RESOURCES |
Category: Refining
There has been rapid technology development in Direct Lithium Extraction (DLE) and this separation technique is being adopted as the standard method for extracting lithium from geological fluids. DLE is part of a larger separation process that includes chemical precipitation, the actual lithium extraction step, media regeneration, and final media rinsing. Since OLI provide technology at the chemical speciation and process chemistry level, it was incumbent upon us to develop capabilities to model this separation. And, since this is essentially an ion exchange reaction, we would try to model it in a mechanistic way.
We wrote in a previous solvent extraction blog, that OLI’s expertise is engineering chemistry and less so, process engineering. We therefore had to learn from clients and from the literature about the DLE process and the media’s mechanism of adsorption. We started this effort in 2019 and have now a semi-empirical database and several case files that clients can use to try out. The text below describes our approach.
Creating a DLE media
The basis of DLE media is a MnO3 or TiO3 powder that contains two equivalents of exchangeable sites in its crystal lattice. These sites can be occupied by H+, Li+, and to a lesser degree other metal ions. Polymers bind the powder to create the media product. Each media supplier creates their own product which has a defined exchange capacity and selectivity coefficients (for the exchange reactions).
We used the existing ion exchange capability in the OLI Databook to create the DLE media. The media has an exchange capacity defined in units of milliequivalents of exchangeable sites /g media (meq/g). We also created exchange reactions between the media and H, Li, Na, K, Mg, and Ca. Each exchange reaction has a selectivity coefficient that is based on the adsorption experiments provided for that media. We divided the exchange reactions for the media into two types. The first, Media(1), adsorbs lithium only. The second, Media(2), adsorbs all cations. This is a practical solution to address the >10:1 adsorption affinity for lithium over other cations.
Below are the exchange reactions we created:
HMedia(1)+ Li+1↔QsLiMedia(1)+H+HMedia1+ Li+1↔QsLiMedia1+H+
HMedia(2)+ Li+1↔QsLiMedia(2)+H+HMedia2+ Li+1↔QsLiMedia2+H+
HMedia(2)+Na+1↔QsNaMedia(2)+H+HMedia2+Na+1↔QsNaMedia2+H+
HMedia(2)+K+1↔QsKMedia(2)+H+HMedia2+K+1↔QsKMedia2+H+
HMedia(2)+0.5Mg+2↔QsMg0.5Media(2)+H+HMedia2+0.5Mg+2↔QsMg0.5Media2+H+
HMedia(2)+0.5Ca+2↔QsCa0.5Media(2)+H+HMedia2+0.5Ca+2↔QsCa0.5Media2+H+
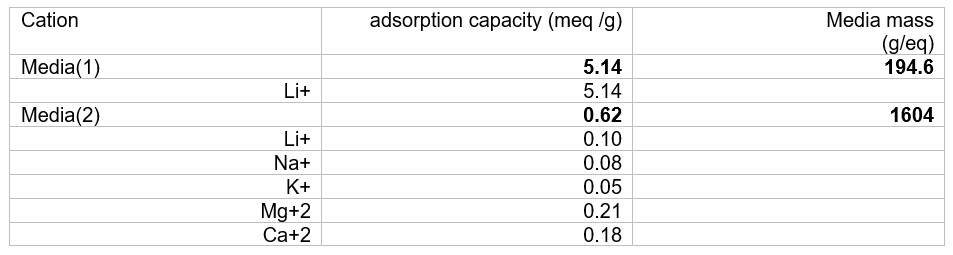
The table below shows how we set the media mass of the media. We used results from Wang et al[i] to create the table below. We back calculated the overall adsorption capacity of the media using the adsorption data reported for each of cation (Li, Na, K, Mg, Ca). The media’s total adsorption capacity is 5.76 meq/g: 5.14 meq/g for Media(1) and 0.62 meq/g for Media(2). This means that Media(1) mass is 194.6 g/mole and Media(2) mass is 1604 g/mole. It assumes that one mole of media adsorbs a cation with one equivalent of charge.
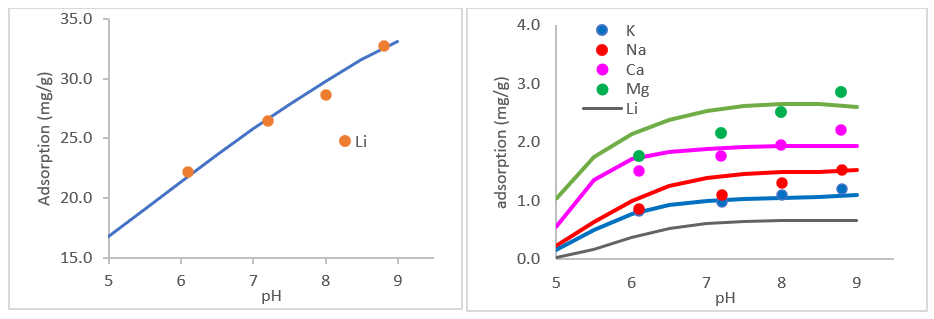
We then used the plots below (same author) to back calculate selectivity coefficients for the six exchange reactions. The left plot fits the selectivity coefficient for Media(1) and the right plot fits the five selectivity coefficients for Media(2). The data are measurements and lines are predictions.
Once we added the Media(1) and Media(2) parameters in the DL1E database, we tested its predictions in the process simulator.
Simulating the DLE process
The DLE process involves four steps, lithium adsorption on the media, media rinsing to remove excess brine, lithium desorption from the media using a strong acid, and a final media rinsing. The initial DLE process used contactors/soakers which acted like single-stage reactors. More recent processes that we have learned about are applying multi-stage column percolation.
Applying DLE to a steady-state process simulator is challenging. Ion exchange is an inherently batch process and works better in a dynamic simulator. But dynamic simulation brings its own set of complexities. Keeping these limitations in mind, we set up a steady state-simulation of the DLE process. The image below shows how this is accomplished. The four boxes represent the four stages of the extraction process. There is a single contactor unit, and this unit is represented by the eight objects shown in the image. If the four-step process takes one hour to complete, then the first box represent the first 25 minutes to adsorb the lithium onto the media, the second box is the five minutes to rinse the media of the brine using fresh water, the third box is the 25 minutes it takes to desorb the lithium using HCl, and the last box is the five minutes to rinse the media of the excess acid.
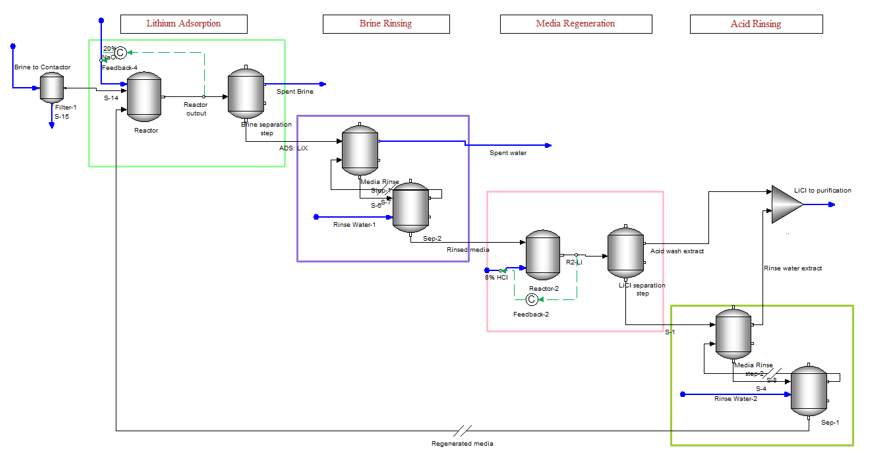
The DLE process is part of a larger plant operation, which may include weak and strong cation exchanger units, high-pressure RO, pH adjustment and filtration of the feed brine, rinse water purification, and chemical mixing. So, the above image is just a part of the larger process.
The table below contains the calculation results: filtered feed brine; spent brine; and LiCl to purification. The LiCl to purification stream is what gets sent to the Li2CO3 or LiOH plant. Using the private database, we can see that Li is the primary cation, but there are other cations in the stream. These cations come from media adsorption and from incomplete media washing (step 2).
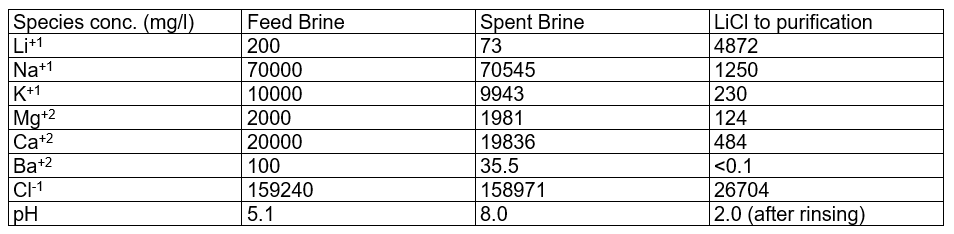
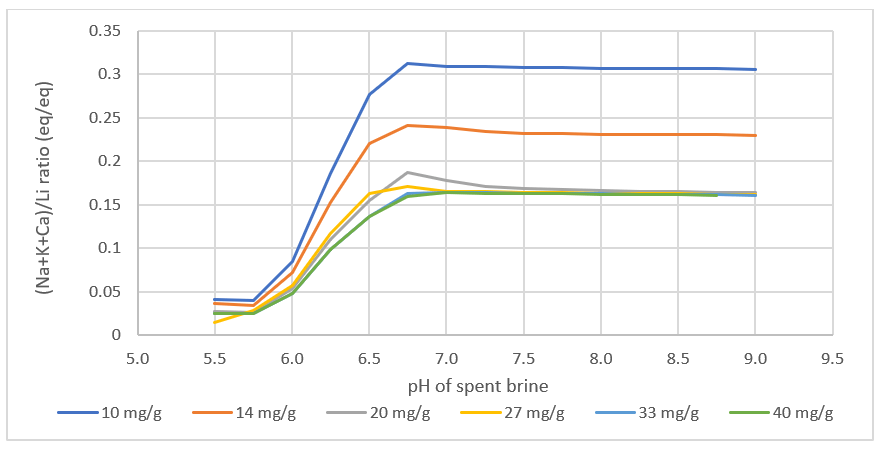
What we found interesting is that the lithium loading, the Li:media ratio in the contactor is computed to impact the product. Since the amount of Na, K, Ca, and Mg >> Li in the feed brine, if the media is underloaded with lithium, meaning that the media adsorbs all the lithium from the brine, then site #2 will adsorb more Na, K, Ca, and Mg, relative to the amount of Li adsorbed. This creates a product brine with more impurities. Also, if the contactor pH is too high, then MgOH2 can precipitate, increasing further the impurities in the final product.
Looking at the results and next steps
This private database was an attempt to quantify the DLE processes being developed by media producers and lithium operators. The database needed to consider the different adsorption site availability for lithium compared to the other cations. The simulation itself needed to consider the complete chemistry, including adsorption/desorption, media washing and dilution, and the precipitation of unwanted salts like Mg(OH)2 and CaCO3.
As the media product become more efficient in removing lithium, we will do our best to keep up with their performance in these private databases, so that our clients can build a credible mass, energy, and chemistry balance of their extraction process.
References
[i] Wang, S. P, Li, X. Zhang, S. Zheng, and Y. Zhang. 2017. Selective adsorption of lithium from high Mg-containing brines using HxTiO3 ion sieve. Hydrometallurgy. Vol 174, pp 21-28. https://doi.org/10.1016/j.hydromet.2017.09.009