RESOURCES |
Category: Refining
Importance of lithium-ion batteries in the development of sustainable energy technology
Lithium has become a strategic mineral that has significant social and environmental implications with the ever-increasing importance of energy storage, which helps mitigate issues of pollution, global warming, and fossil-fuel shortage. The development of lithium-ion batteries (LIBs) has transformed electric technology by virtue of their power as lightweight and rechargeable batteries that could store large amounts of energy to power everything from mobile devices to laptop computers and electric vehicles, making possible a fossil fuel-free society. Compared to other high-quality rechargeable battery technologies such as those with nickel-based batteries, Li-ion batteries offer distinct advantages of providing high energy densities, high working voltage, low maintenance, low self-discharge rate, no memory effect, and nontoxic. These advantages have also made lithium-ion batteries the attractive power sources for military and aerospace applications. The Li-ion battery technology, although facing many challenges in its advancement [1], has become a technology of choice in building reliable and clean energy systems of the future.
Electrolytes in lithium-ion batteries
A typical lithium-ion battery consists of a negative graphite-based anode and a positive Li-containing metal oxide cathode (e.g., LiCoO2), separated by a Li-based electrolyte. Figure 1 is a schematic diagram of lithium-ion battery.
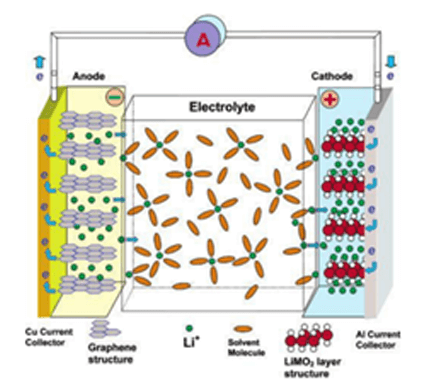
Figure 1. Schematic diagram of lithium-ion battery (LIB) consisting of the cathode (Li-intercalation compound) and anode (graphitic carbon) separated by electrolyte [2].
Electrolytes in a lithium battery are in close interaction with both electrodes and play a crucial role for its electrochemistry. They provide the ion-conductive medium where the Li+ shuttles between the electrodes. The important features of electrolytes required for battery applications include (1) high electrical conductivity and low viscosity for effective ion transport; (2) wide electrochemical window so that electrolyte degradation would not occur within the range of the working potentials of both cathode and anode; (3) high solubility and wide thermodynamic liquid range which require low melting and high boiling temperatures; (4) inert to other cell components and low chemical reactivity; (5) safe, non-toxic, and economical.
Among several types of battery electrolytes (i.e., aqueous, non-aqueous and solid), the non-aqueous electrolytes containing lithium salts in aprotic organic solvents balance the requirements for LIBs and become the state-of-the-art electrolytes commonly used in commercial lithium-ion batteries. Aprotic solvents can avoid hydrogen evolution due to the lack of active protons and are chemically stable toward lithium. Based on the required features for battery electrolytes, organic carbonates and their mixtures, particularly those of ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and diethyl carbonate (DEC) have been found to be the most suitable solvents for lithium-ion batteries. For the lithium salts,
some of the important properties include high dissociation constants and high ionic mobility (especially Li+) in nonaqueous media, non-toxicity and stability of the anion against redox (at electrodes) and thermally induced reactions with electrolyte solvents and other cell components. The most commonly used lithium salt is LiPF6, as it offers the required properties better compared to other salts such as LiBF4, LiClO4, and LiAsF6, which are among widely investigated salts for LIB electrolyte applications [2].
Importance of thermophysical property modelling in LIB performance improvement
Properties of the solvent and electrolyte in battery applications can be tuned by mixing two or more of the carbonates and adjusting their compositions and salt concentrations. Composition and ratio of these carbonates have important implications for energy density, cycle life and the safety of lithium-ion batteries; the best ionic conductivity can be achieved through adjusting the salt concentration together with solvent composition. To ensure proper design and operation of the lithium-ion batteries, the battery electrolyte must be within liquid range; at the same time, it should provide good electrical conductance. A combined use of thermodynamic model with a predictive electrical conductivity model can guide the design of the battery electrolyte systems to achieve its optimal operating conditions. The thermodynamic model predicts phase equilibria to define conditions where the system is within the liquid range, whereas the electrical conductivity model predicts the variations of electrical conductivity with temperature, solvent composition, and salt concentration, so that it can pinpoint the operating conditions under which good conductivities are reached. These models play an important role in the development and quality control of lithium-ion batteries.
OLI simulation tools for predicting phase equilibria and electrical conductivities for LIBs
OLI Systems, Inc. has developed advanced technologies based on its proprietary thermodynamic model, the Mixed-Solvent Electrolyte (MSE) model [3] and the electrical conductivity model built upon the MSE model [4]. These models, which are incorporated in OLI software Platform V11.5, can be applied to non-aqueous electrolyte systems pertinent to lithium-ion battery applications. These models can be used to interpret behaviours of key variables leading to the improved Li-ion battery performance. For example, the MSE thermodynamic model can predict the solid-liquid equilibria which define appropriate operating (liquid) range; this information can then be combined with electrical conductivities determined from the conductivity model for a comprehensive analysis for the LIB electrolyte systems to provide insights for the LIB design and operation.
The current OLI simulation tools provide LIB-related electrolyte predictions for systems containing:
- Organic carbonates
- diethyl carbonate (DEC)
- dimethyl carbonate (DMC)
- ethylene carbonate (EC)
- propylene carbonate (PC)
- Lithium salts LiPF6, LiBF4, LiClO4
How reliable is OLI’s simulation for predicting LIB electrolyte properties?
The OLI models for the lithium battery electrolyte systems have been developed based on the analysis of the experimental data including solubilities, melting, and boiling point temperatures, heat capacities, heats of mixing, densities, electrical conductivities. These data are critically evaluated for their dependencies on temperature, solvent composition and lithium salt concentrations. The comprehensively calibrated models provide predictions for the phase equilibria and electrical conductivities that are in good agreement with experimental results. The comparison of the experimental solid-liquid equilibrium data with results from the MSE model for the LiBF4+DMC mixture is shown in Fig. 2. Addition of LiBF4 to solvent DMC results in decreased solvent melting temperatures in the salt mole-fraction range of 0~0.3 (or 0~4.7 mol/kg solvent), thus expanding the liquid stability range to lower temperatures. The experimental results are accurately represented by the MSE model.
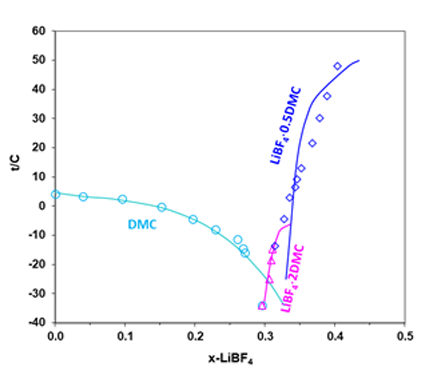
Figure 2. Solubility in the LiBF4 + DMC system (Ref: Plakhotnik, et al. 2004)
The MSE model can also predict phase behavior for the LIB electrolyte systems when solvent is a mixture of organic carbonates. Demonstrated in Fig. 3 are the comparisons of calculated melting temperatures of EC and DMC with experimental data. In this figure, phase diagrams for EC + DMC and for EC + DMC + 1.0 M LiPF6 are shown.
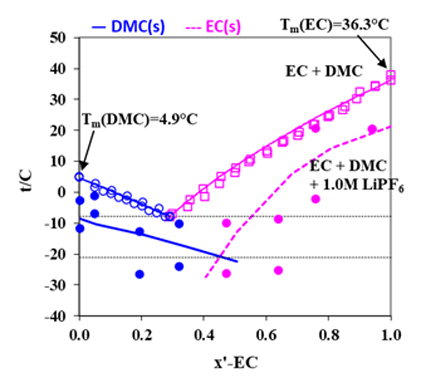
Figure 3. Phase diagram for EC + DMC with (lower lines) and without (upper lines) LiPF6 (Refs: Ding, et al. 2000, 2004; Tarascon et al., 1994; Stewart et al., 2008)
Like the LiBF4 + DMC system, addition of 1.0 M lithium salt, LiPF6, to the mixture of EC+DMC is seen to result in decreased melting temperatures, leading to an increased liquid stability range in the non-aqueous electrolyte system, making possible for the LIB to operate at lower temperatures. These behaviors are reasonably reproduced by the MSE model within the uncertainties of the experimental data. For such systems, the electrical conductivities can be predicted using the conductivity model, as illustrated in Fig. 4 for the LiPF6 + DEC + PC system. The electrical conductivities reach a maximum at a LiPF6 concentration of approximately 1M. Figure 5 shows solvent composition (as x-EC, x is mole fraction) effects on the conductivity in the LiPF6 + DMC + EC system at various temperatures and at the LiPF6 concentration of 1.0 M. The electrical conductivities are accurately predicted for these systems using the model. These results can provide strong foundation for the LIB design and performance improvement.
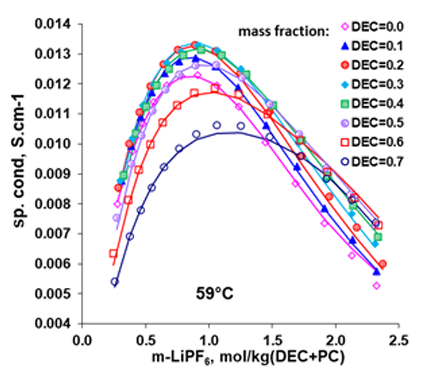
Figure 4. Electrical conductivities in LiPF6 + DEC + PC mixtures at various DEC:PC ratio as a function of LiPF6 concentration at 59°C (Ref: Ding, 2003).
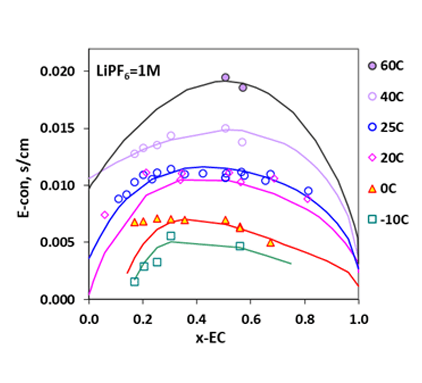
Figure 5. Electrical conductivities in LiPF6 + DMC + EC at various temperatures as a function of solvent composition (as mole-fraction of EC) at 1.0 M LiPF6 (Refs: Xiao et al. 2004; Tarascon et al.,1994; Christie et al. 1998; Schmidt et al. 2001; Hayashi et al. 1999).
What tools are available for predicting LIB electrolyte properties?
The OLI System’s thermophysical property package, which implements the MSE thermodynamic model, and the transport property models, with the capability of predicting LIB electrolyte phase equilibria and electrical conductivity, is available in OLI Studio and OLI Flowsheet ESP.
References
- Hannan M. A., et al., Renewable and Sustainable Energy Reviews 69 (2017) 771–789
- Xu K., Chemical Reviews, 104 (2004), 4303-4417
- Wang P., Anderko A., Young R. D., Fluid Phase Equilibria 203 (2002) 141-176
- Wang P., Anderko A., Young R. D., Industrial & Engineering Chemistry Research 43 (2004) 8083-8092










