RESOURCES | post
Category: Water & Wastewater Treatment
REEs recovery processes need innovation
Over the last few decades, a group of 17 elements composed of scandium, yttrium, and the lanthanides called “the rare earth metals”, have become the key to designing and developing high technology products used in our daily lives ranging across consumer electronics devices, automotive components, rechargeable batteries, and medical imaging. A diverse set of applications of the rare earth elements (REE) in the modern industry has led to a surge in demand as exemplified by an increase in global mine production to 210,000 tons of rare-earth-oxide equivalent in 2019, an 11% rise compared with that of 2018. The estimated value of rare-earth compounds and metals imported by the United States increased approximately 6.25% in 2019. Although the REEs are relatively abundant in the Earth’s crust, they are difficult to mine as REEs are not found in large quantities or in veins like other minerals such as gold. Nowadays, more than 250 rare earth minerals have been recognized, but in most of them, the concentration of rare earths is very low, varying from 10 to 300 ppm. The principal economic sources of rare earths are the minerals bastnaesite, monazite, and loparite and the lateritic ion-adsorption clays. In the absence of any primary operational deposits, many countries are forced to rely on secondary sources such as recycling of rare earth metals from pre-consumer scrap, industrial residues, and end-of-life products. One such widely recognized secondary source for REEs is phosphogypsum, a pollutant waste generated by the phosphoric fertilizer industry. Managing this pollutant is challenging due to the large volumes generated worldwide. A promising way of extracting value out of phosphogypsum is to recover rare earth elements. However, an optimized recovery scheme is essential to create any such cost-effective and environmentally friendly process. Conventional methods to extract rare earth elements from ores generate millions of tons of toxic and acidic pollutants. But instead of using harsh chemicals, a process can be conceptualized using bio-lixiviants to extract the elements from phosphogypsum.
How can thermodynamics help to design and optimize the recovery of REEs?
Thermodynamic simulations are essential for achieving optimum conditions for various processing techniques (e.g., REE leaching or deposition) needed to maximize product recovery. Thermodynamic analysis can help to identify the most promising combinations of lixiviants, rare earths and process conditions for the separation through solubilization or precipitation of REEs. It allows us to explore environmentally benign chemical alternatives such as bio-lixiviants that can achieve optimal extraction over a specific range of pH based on different solubility parameters. In effect, thermodynamic simulations can be demonstrated as an invaluable tool for new process discovery. It can translate the results of laboratory experiments to real-world process conditions, in which the complexity and variability of feedstocks may affect the effectiveness of REE recovery. Over the last several years, OLI Systems Inc. has been at the forefront of discovering environmentally friendly cutting-edge separation techniques for the recovery of REEs from secondary sources under the auspices of the Department of Energy’s Critical Materials Institute (CMI) research hub. In some recent significant contributions, OLI Systems, in collaboration with Rutgers University, has outlined environment-friendly processes for the recovery of REEs. Examples include phosphogypsum leaching using various lixiviants, including a bio-lixiviant for the recovery of REEs and separation of REEs as corresponding REE carbonate phases using monoethanolamine (MEA)-CO2 solutions. [1, 2]
Can we reliably predict the thermodynamic properties of REEs using OLI software platform?
The MSE thermodynamic framework in the OLI software can accurately estimate solubilities of rare earth chlorides, sulfates, fluorides, hydroxides, carbonates, and citrates over wide ranges of process conditions such as pH and temperature in a complex multi-component mixture while identifying stable and meta-stable hydrated and anhydrous solid phases. For example, Figure 1 gives the solubility behavior of neodymium carbonate as a function of solution pH at temperatures ranging from 25 to 150 °C which is in good agreement with the experimental data measured in CO2 atmosphere mostly in the acidic and near-neutral pH range. In general, the solubilities of Nd2(CO3)2 and NdOHCO3 are very close in the acidic and near-neutral pH range, which indicates that they have a similar tendency for precipitation. Nd2(CO3)3 is thermodynamically more stable (i.e., it has a slightly lower solubility) at lower pH within a very narrow pH range, followed by similar stability of both Nd2(CO3)3 and NdOHCO3 at slightly higher pH. However, in the basic pH region, NdOHCO3 is predicted to be more stable than Nd2(CO3)3. This is due to the effect of increasing concentration of OH– ions on solid–liquid equilibria.
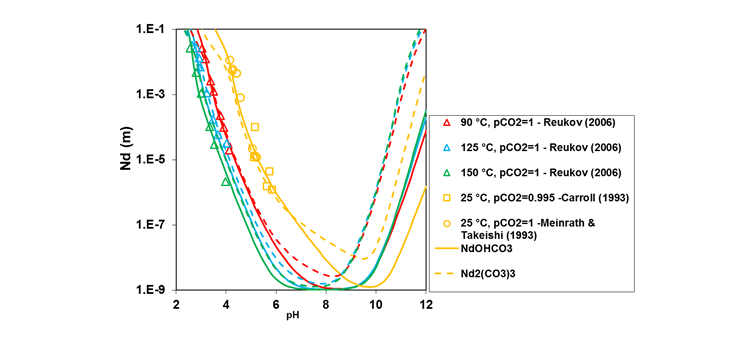
Figure 1. Nd2(CO3)3 and NdOHCO3 solubilities as a function of pH in CO2 atmosphere with pCO2 = 1 at 25-150 °C
Having established the thermodynamic foundation as shown in Figure 1, the model can be now applied to simulating an actual process. Figure 2 represents simulation results for the NdCl3-MEA-H2O-CO2 system as yield diagrams. The yield diagrams elucidate the concentrations of reactants that are conducive to attaining the 0.99 fractional yield of the desired carbonate phase (green area). The predicted yield fraction serves as a measure of the effectiveness of the separation procedure and is obtained by dividing the amount of the desired product calculated according to thermodynamic equilibrium by the amount that would be obtained if all of the available neodymium was stoichiometrically converted to either Nd(OH)CO3 (cf. upper diagram) and Nd2(CO3)3 (cf. lower diagram). In the scenario studied here, the reaction between MEA (0-3 molal) and NdCl3 (10-8-1 molal) in the presence of CO2 (1 atm) at 25 °C result in the formation of neodymium carbonate phases.
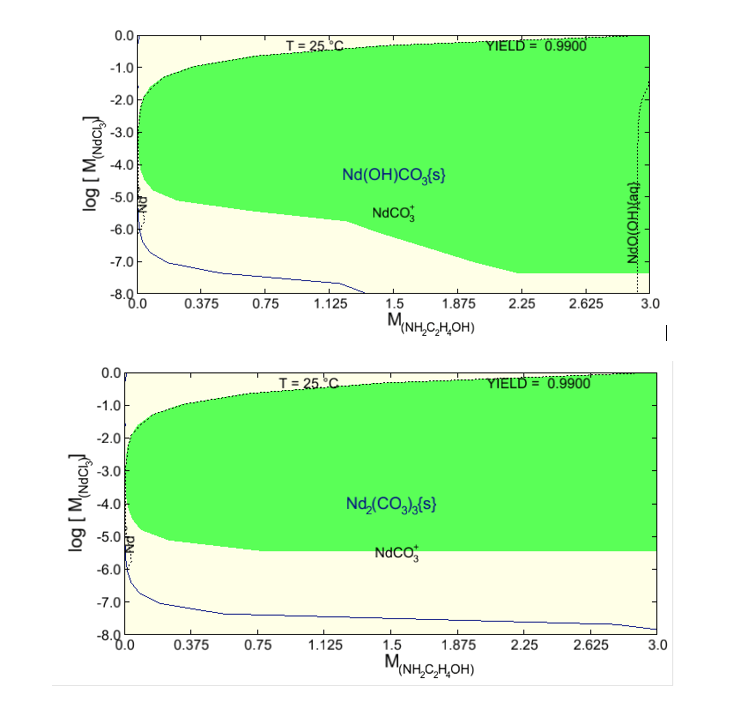
Figure 2. Yield diagrams for the formation of the stable NdOHCO3 phase (upper diagram) and the metastable Nd2(CO3)3 phase (lower diagram) at 25 °C as a function of reactant (NdCl3, MEA) concentrations at 1 atm CO2. The shaded regions indicate 99% yield of the desired NdOHCO3 and Nd2(CO3)3 phases
OLI software is useful for identifying the most efficient leachate amongst all readily available lixiviants for REE extraction over a certain range of pH conditions. The following figure (Figure 3) shows the solubilities of neodymium at the natural pH of 220 mM acid solutions. The pH of a 220 mM gluconic acid solution, a predominant component in bio-lixiviants, is 2.1 whereas the natural pH of the mineral acids is substantially lower due to the higher strength of these acids. Thus, the solubility in 220 mM acid solutions results from the combination of the pH effect and the specific complexation/common ion effects. As shown in the figure, the predicted solubility in 220 mM acids follows the order sulfuric acid > gluconic acid > phosphoric acid. This order is consistent with the experimentally observed effectiveness of the lixiviants. Such fundamental thermodynamic analysis is important in developing a comprehensive mapping of lixiviants and demonstrates their effectiveness in solubilizing various mixtures of rare earth hydroxides.
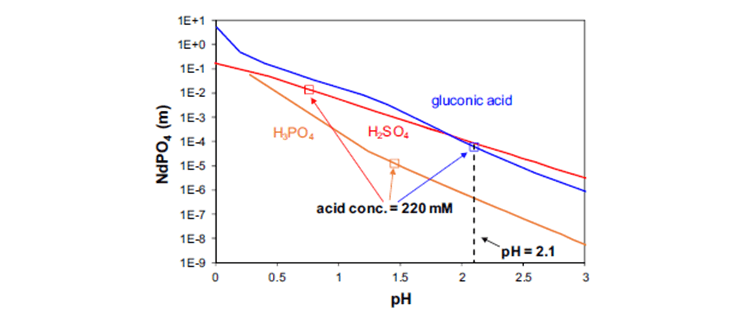
Figure. 3 Solubility of NdPO4 in gluconic acid (blue line), sulfuric acid (red line), and phosphoric acid (orange line) as a function of pH. The square symbols show the solubilities in the three acids at the natural pH of 220 mM solutions.
What tools are available for predicting REE properties?
The OLI System’s thermodynamic property package, which is based on the MSE model, is available in OLI Studio and OLI Flowsheet: ESP.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- Antonick, P.J., Hu, Z., Fujita, Y., Reed, D.W., Das, G., Wu, L., Shivaramaiah, R., Kim, P., Eslamimanesh, A., Lencka, M.M. & Jiao, Y., Bio-and mineral acid leaching of rare earth elements from synthetic phosphogypsum. The Journal of Chemical Thermodynamics, 2019, 132, 491-496.
- Kim, P., Das, G., Lencka, M. M., Anderko, A., & Riman, R. E., Earth Element Recovery Using Monoethanolamine. Journal of Materials Engineering and Performance, 2020, in press.
- Das, G., Lencka, M. M., Eslamimanesh, A., Wang, P., Anderko, A., Riman, R. E., & Navrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. The Journal of Chemical Thermodynamics, 2019, 131, 49-79
- [Das, G., Lencka, M. M., Eslamimanesh, A., Anderko, A., & Riman, R. E. Rare-earth elements in aqueous chloride systems: Thermodynamic modeling of binary and multicomponent systems in wide concentration ranges. Fluid Phase Equilibria, 2017, 452, 16-57









