What is pH?
pH is a scale used to specify the acidity or basicity of a solution. Acidic solutions have lower pH values than basic or alkaline solutions. The pH scale is logarithmic and inversely equals to the activity of hydrogen ions in the solution (pH =-log(aH+)). pH is an important quantity as it can reflect the chemical and biochemical properties of a solution. pH can be important in many branches of science such as biology, chemistry, geology, environmental, etc. as well different segments of industry, such as water and wastewater treatment, agriculture, nuclear, oil and gas, CO2 capture and storage, etc.
Why is fluid pH important in the oil and gas industry?
During the production of oil and gas large amounts of water (brines) are produced. The produced water is separated from the hydrocarbon phase before the production stream is transferred to refineries. Additionally, high amounts of water are usually used as injection water in hydrocarbon enhanced recovery processes. Down the line, in refineries, large volumes of water are used in boilers and other equipment for the separation of different hydrocarbons in distillation columns. pH as a parameter, that provides information about the chemical composition of these waters and can be measured readily, plays a substantial role in many material selection, operational, and maintenance decisions in the above-mentioned processes. Some examples for pH-related concerns in oil and gas industry are: formation damage, scale precipitation, corrosion of tubulars, rig equipment, and other metallic parts, efficiency of corrosion inhibitors, scale inhibitors, surfactants, and other additives, rig-site filtration efficiency, oil emulsification, etc. Among these, the connection between fluid pH and corrosion problems can be more concerned to operators. Produced waters contain dissolved gases such as carbon dioxide (CO2) and hydrogen sulfide (H2S). These dissolved gases induce uniform and/or localized corrosion, sometimes hydrogen embrittlement and cracking to metallic parts and equipment. The risk of damage is strongly related to in-situ fluid pH. Therefore, accurate determination of fluid pH and adjustments are very crucial to asset design and integrity and operational excellence in the oil and gas industry.

Figure 1. Produced water samples with different water to oil ratios.
Why is accurate prediction of fluid pH important in the oil and gas industry?
pH measurement is not always an easy and/or accurate task in the oil and gas industry due to various reasons. First, the presence of hydrocarbons, high ionic concentrations, wax deposits, and microorganisms contaminate the pH probes. Second, sometimes measuring pH is difficult or not possible, for example, in downhole where it is inaccessible, or at high temperatures where pH probes do not result in accurate readings, or at top of the pipelines where small droplets of water are produced because of condensation. Third, the measured pH obtained from the samples are not always the same as in-situ pH values, for instance, when a sample is exposed to the atmosphere the chemical composition of the sample varies gradually because dissolved gas molecules leave the solution. Hence, accurate prediction of pH helps to overcome the challenges associated with pH measurement in the oil and gas industry.
Why is the OLI software ideal for fluid pH prediction?
Several models exist for predicting pH in operational conditions often seen in the oil and gas industry. However, most predictions tools are limited to relatively low temperatures (< 150oC), low pressures (< 50 bar), and to slightly concentrated brines (up to 1 mol/lit) solutions. Some of these models are simple empirical equations that allow calculating the chemical speciation in a solution as function of temperature, pressure, and ionic strength, for example, Oddo and Tomson model, and Millero et al. model. Another group of models is more advanced and considers the interspecies interactions between dissolved species in a solution, e.g., Pitzer model. The mixed-solvent electrolyte (MSE) model is a comprehensive semi-empirical model that can predict the phase and chemical equilibria as well as thermal and volumetric properties of water, nonaqueous, or mixed solvents containing electrolytes and non-electrolytes with concentrations ranging from dilute solutions to highly concentrated solutions.
The MSE model incorporated in the OLI software covers a wide range of temperatures (up to 300oC), pressures (up to 3500 bar), and concentrations from very dilute solutions, i.e., pure water, to fused salts. Moreover, the MSE model considers the interactions between species in the liquid phase as well as in the gas phase. Last but not least, the number of species included in the MSE model is much higher than any other available platform in the open literature and in the market. All of these features make the MSE model the most accurate model for the prediction of pH in the oil and gas industry.
In the following two examples, pH prediction by OLI software for CO2 and H2S saturated aqueous solutions frequently encountered in the oil and gas industry are presented. The dots and bars are experimental data, and the solid lines are predicted pH values obtained by the OLI software. Figure 1 shows pH as a function of CO2 gas partial pressure at different temperatures.
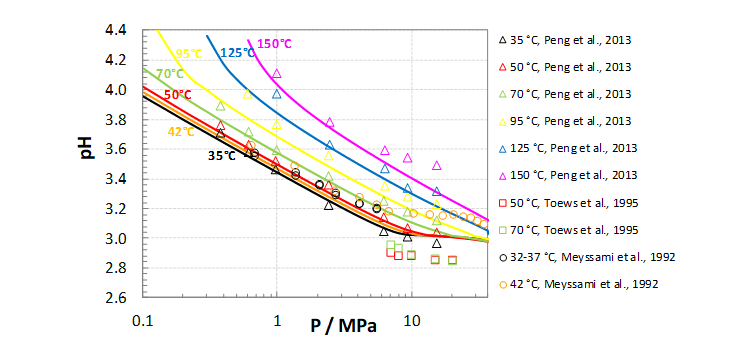
Figure 2. pH vs. total pressure for the H2O rich phase in an H2O-CO2 open system
Figure 2 shows pH values vs. NaCl concentration for two systems of H2O-CO2-NaCl system and H2O-H2S-NaCl at 25oC and 1 bar total pressure.
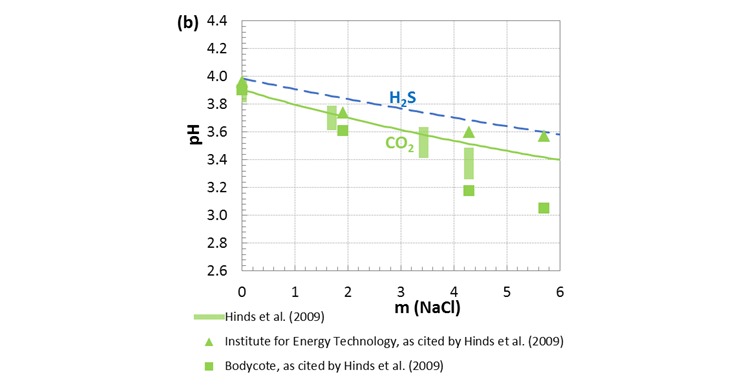
Figure 3. pH vs. NaCl concentration in H2O-NaCl-CO2 and H2O-NaCl-H2S open systems at 25oC and 1 atm total pressure.
The predicted pH values agree well with the experimental data in both examples, demonstrating that the MSE model can provide an efficient and reliable tool to accurately predict pH in the oil and gas industry.
What tools are available in the OLI software for predicting pH?
pH can be predicted by the OLI System’s thermodynamic property package, which implements the MSE and MSE-SRK models. The pH prediction tools are available in OLI Studio, OLI Flowsheet ESP, and in Web APIs. For more information about pH prediction tools in the OLI software contact OLI to schedule a meeting with an OLI expert.
For more information on the OLI Studio software Platform please visit https://www.olisystems.com/software/oli-studio/; for more information OLI’s corrosion management capabilities, please visit https://www.olisystems.com/solutions/aqueous-corrosion-management/.
To speak to an OLI technical or sales specialist please register your interest at https://www.olisystems.com/contact-us/ or send an email to [email protected].
References
- Springer R.D., Wang P., Anderko A., SPE Journal (2015) Paper No. 173902.
- Plennevaux C., Ferrando N., Kittel J., Fregonese M., Normand B., CORROSION Conference (2013) Paper No. 2843, Orlando, FL, USA.
- Oddo J.E., Tomson M.B., J. Pet. Technol. 34 (1982) 1583–1590.
- Millero F.J., Geochim. Cosmochim. Acta. 59 (1995) 661–677.
- Millero F.J., Hershey J.P., American Chemical Society (1989) 282–313.
- Felmy A.R., Weare J.H., Geochim. Cosmochim. Acta. 50 (1986) 2771–2783.
- Pitzer K.S., Activity Coefficients in Electrolyte Solutions, 2nd ed., CRC Press, 2018.
- Pitzer K.S., J. Am. Chem. Soc. 102 (1980) 2902–2906.
- Hinds G., Cooling P., Wain A., Zhou S., Turnbull A., CORROSION, 65 (2009) 635–638.