Rare earth elements has been a hot topic ever since the geopolitical crisis of 2010 when China, which supplied ~90% of global demand, restricted exports. The net result was a 10-100x spike in prices, and a scramble by countries to find domestic supplies. Since then, the non-China production has increased to ~30%.
The Rare earth elements market is not large, about 350 kt/yr and $6bn/yr in 2023/2024. But these elements are essential ingredients for permanent magnets, photovoltaics, fuel cells, and other critical products. Also, their value is expected to grow to $11bn by 2029.
The responsibility of technology companies like OLI is to help companies increase their production. And, specific to OLI, to uncover potential hydrometallurgical pathways to extract these metals from non-traditional sources.
What are Rare earth elements
The Rare earth elements are the fifteen lanthanide metals found at the bottom of the periodic table. Grouped with the fifteen lanthanides are yttrium (Y) and scandium (Sc). One of the lanthanides, promethium (Pm), is generally excluded from hydrometallurgical processing. So, that leaves sixteen elements that go by the abbreviation REE or REY (the latter emphasize that yttrium is included).
Most REE are mined from two sources, REE-bearing minerals and from REE-adsorbed clays. These operations have existed for years, and their process is well established. These source however, have limited reserves and are geographically localized. So, future production will rely on alternative sources, especially when a country’s goal is to secure a domestic supply.
The likely candidate resources include phosphogypsum, coal ash, and coal gangue. These “discards” are the by-product of phosphoric acid production and power generation and contain anywhere from 0.01 to 0.2 wt% REE. This concentration is much smaller than existing ores (>>1%), but the sheer quantity of material makes them potential sources. For instance, the quantity of REE discarded during phosphoric acid production in Florida (USA) is conservatively 30 kt/y, or about 10% of global production. The annual mass of REE discarded from coal burning is ~400 kt/y, greater than total global demand. And, since these waste materials have been discarded for decades, the potential quantity is in the millions of tonnes. The University of Texas (go Horns!) for instance, estimated that 11MM tons of REE is contained in coal ash alone, 40x the annual global demand.
So, resource recovery from waste materials is a potential opportunity, if we can figure out how to extract it. That’s what we’ll discuss below.
Opportunity – Phosphogypsum
I want to focus on the promise of extracting REE from phosphogypsum (PG). PG is produced by dissolving apatite ore (calcium phosphate) in sulfuric acid. The discarded solids is the PG. Apatite ore is not pure, and so the discarded PG will contain minor concentrations of elements like Al, Fe, F, U, Th, Ra, and REE. The REE is the prize, and this is where OLI technology attempts to make a contribution..
OLI’s current capabilities in REE
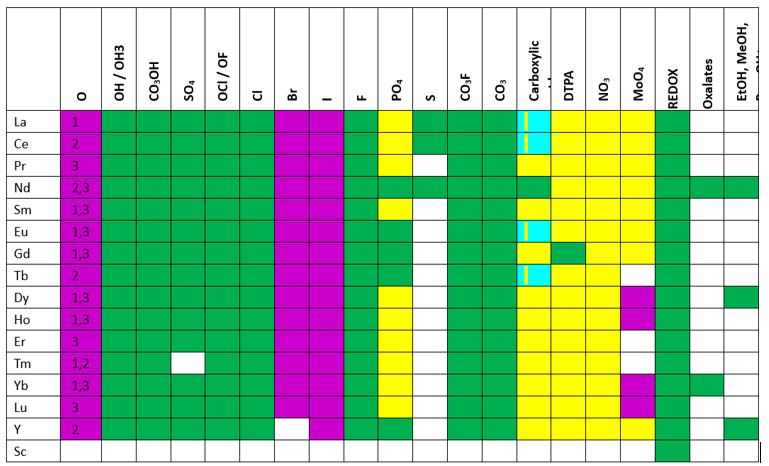
OLI started work in REE thermodynamics in 2011 with a goal of predicting their properties during hydrometallurgical operations. A significant portion of this work was completed by my colleague, Dr. Gaurav Das, and he published several peer-reviewed papers on the topic. The chart below shows OLI’s progress as of May 2024. The green cells show where the chemistry is studied thoroughly. The yellow cells are where we currently estimate properties, and the white are what is incomplete.
So, our credentials lay in predicting the behavior of REE’s in process environments. And that’s where this blogs is going. If we want to design a PG extraction process, what can we expect when the acid reacts with the PG, and how does the REE leach- or dissolve-out of the PG? The answer is not straightforward, and this is the story as we understand so far.
Looking at the composition of Phosphogypsum
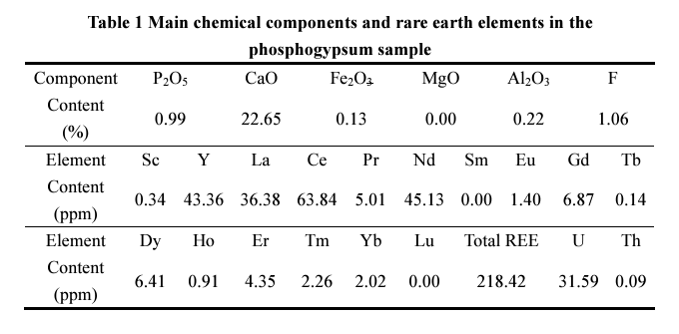
A group of researchers at Florida Polytechnic University studied the PG waste deposits in Florida (USA). They summarized the PG composition in the table below. This PG sample contains 218 ppm REE distributed among eleven elements, Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu.
The authors also performed several extraction tests, one of which is a plot showing the fraction of the REE released as they varied the ratio of 5% H2SO4 to PG (H2SO4:PG). As H2SO4:PG increased, the fraction of PG recovered increased as expected. But, only about 50% of the REE leached out at seven (7) parts H2SO4 to one part PG.
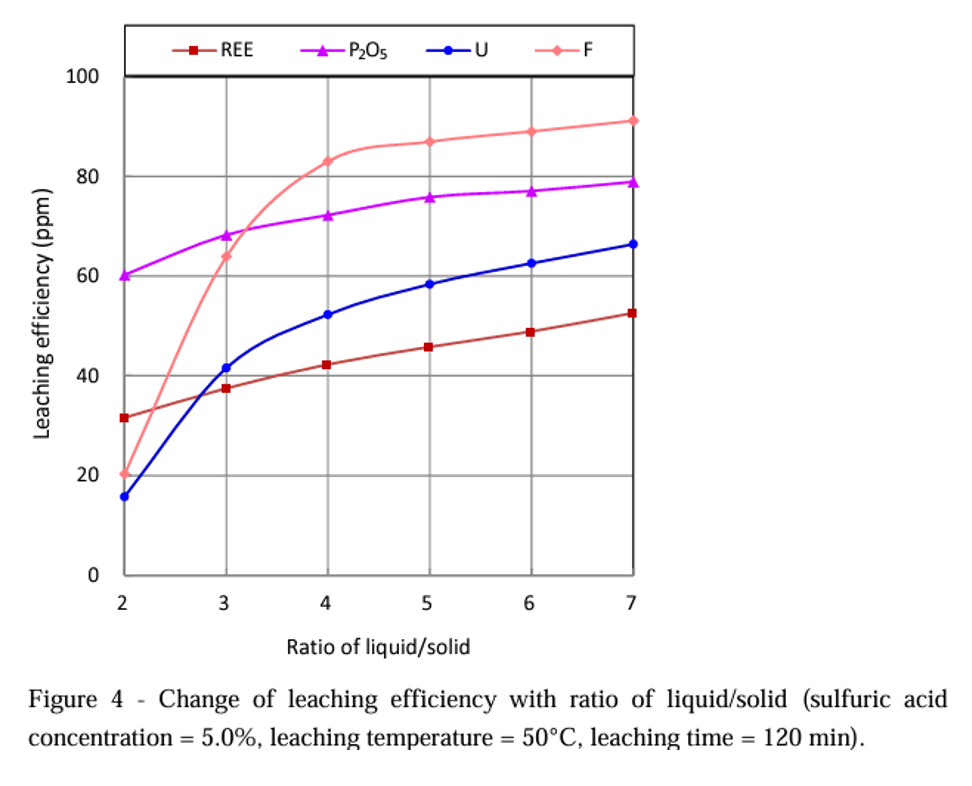
The next step is to verify current predictions using this data and then design an extraction process around it.
Predicting the experiment so that we can design the process
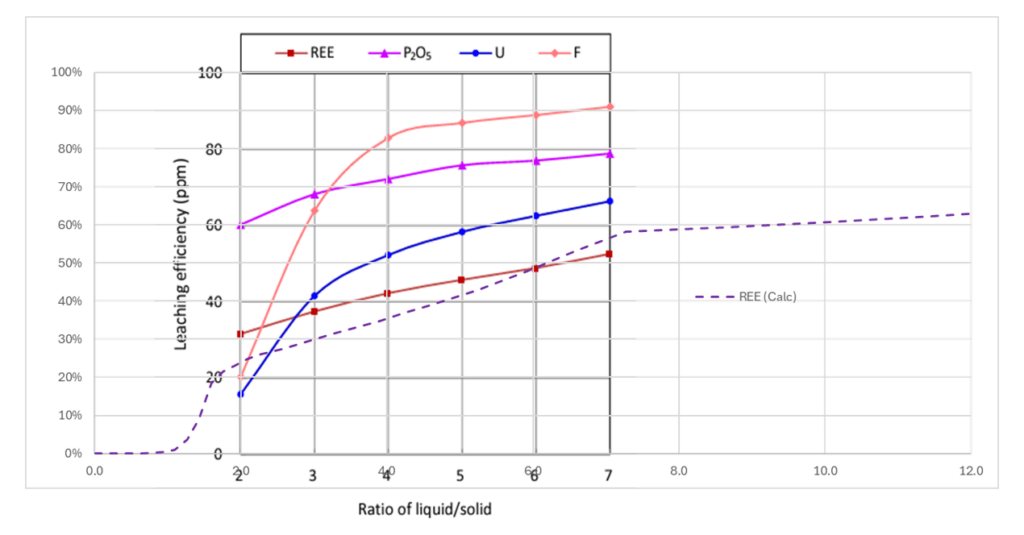
We created a leaching simulation inside OLI Studio using the above chemical analysis. We then created a private database and added some of the RE-PO4 salts that were missing in the main database (we used estimates for their solubilities). We then guessed the portion of REE that is found embedded in the gypsum solid and the portion that is present as separate phases. We also estimated, and in some cases fit, the solubility of some RE-PO4, because they were missing from the main database. The plot below shows the extraction predictions against the measured data. The predictions were good enough to investigate further.
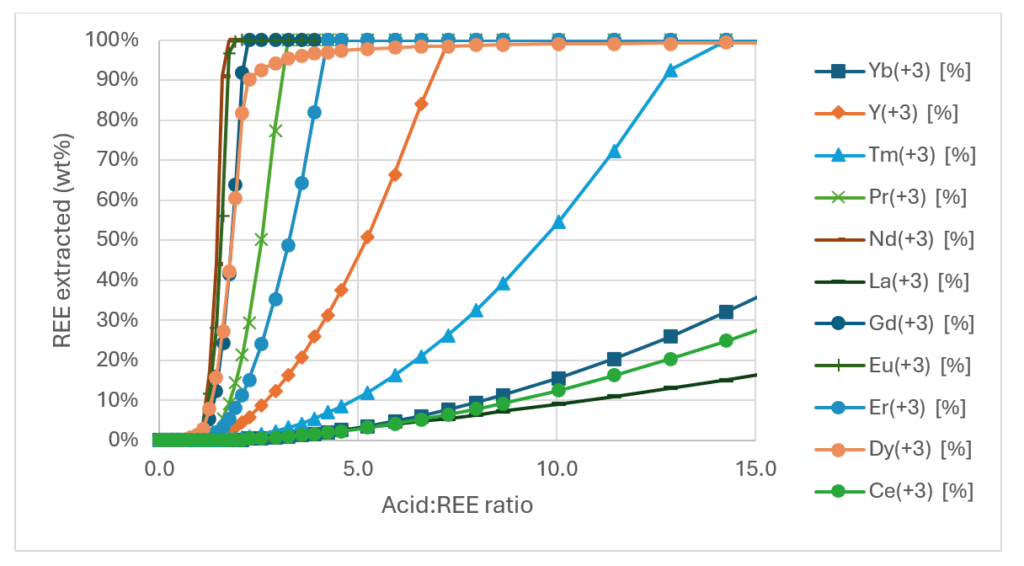
Knowing that the solubility of each REPO4 and REF3 varies, we investigated if the model predicted REE separation during the leaching experiment. That is what the next plot shows. It’s a busy plot, so we’ll review it in groups. A group of three elements leach completely at H2SO4:PG<2. A second group of four elements leach out moderately when the H2SO4:PG is between 3 and 12. A third group of three leach gradually when the H2SO4:Acid is between 5 and 15.
Converting to a process design
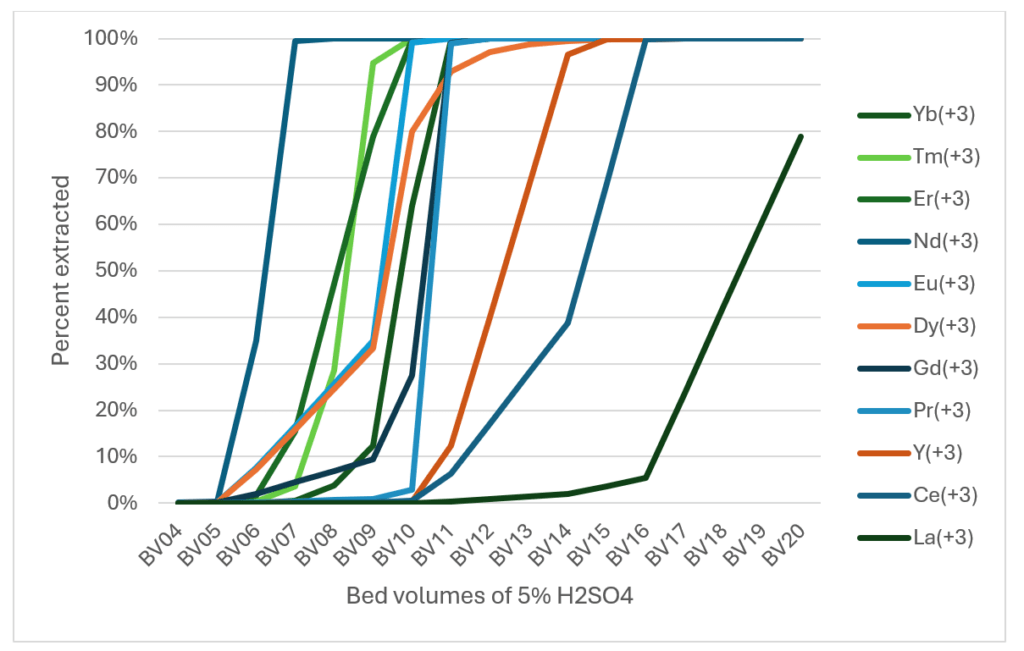
If these extractions are qualitatively correct, then we can treat the process like a fractionation column. The PG heap would be stacked to a sufficient height to produce enough fractional separation. We would then continuously percolate bed volumes of acid through the heap. As each bed volume (BV) eluted would be processed to separate the REE that is already semi-purified.
The plot below shows how the REE would extracts with each BV of acid. Again, a lot of lines, but what seems clear is that we could use a fractionation process to extract the elements with some initial separation.
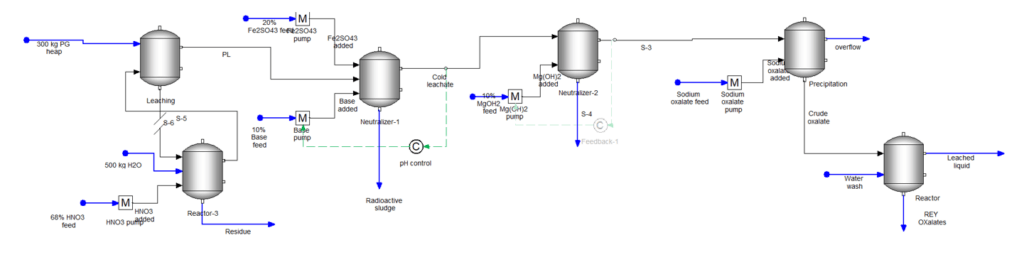
All this is based on understanding a few things. The first is how the PG incorporates the REE and the second is how the REE reacts once mobilized. Will the elements elute with the acid or do they precipitate as phosphate or fluoride salts? With additional detail about how the REE is affixed to the PG, with additional work to improve the solubility database (this second part is important), and with adding some transport parameters, we could design a process like the image shown below, that effectively produces REE from this waste resource. This can be a topic for a follow-up blog.