Approximately thirty years ago, a Joint Industry Program (JIP) was formed to develop technology needed to produce natural gas where methane hydrate form. The project, Deepstar, studied among other topics thermodynamic hydrate inhibitors. OLI’s contribution was to develop a thermodynamic database that predicted methanol reactivity with the produced brine and gas. In the mid-2000’s, OLI extended the database to predict ethylene glycol (MEG) properties in the same brine-gas fluids.
Each technology company in this space contributed to this effort because natural gas production in deepwater environments represented an important energy resource. Our contribution was to develop software that predicted the properties of these fluids from the injection point to the regeneration process. This contribution helps engineers design the best treatment and regeneration system on the production platform.
As with other blogs we write, our focus is on engineering-chemistry; that is, the composition and properties of the fluids being produced. In this blog, we want to present the interesting properties of MEG regeneration, since maximizing its recovery is a key economic criterion to operations.
We will focus on the following items in this blog, the solubility of brine salts in MEG-H2O mixtures, the fluid properties when MEG is separated from H2O and salts, and the impact of dissolved salts, temperature, and pressure on phase separation.
The properties of MEG-H2O mixtures
It might be worthwhile to start with the importance of MEG on the properties of water, since this is the key property for gas-hydrates. The plot below shows the freezing point water as MEG is added. The physical-chemical effect of adding MEG is to disrupt the bonding between H2O molecules. When this happens, temperatures need to be lowered (heat removed), so that the chemical bonds that hold the ice-crystal together exceed the ability for vibrating water molecule to break it. This is the general principle of MEG inhibition, change the thermodynamic properties of the H2O-CH4 mixture so that a solid phase does not form. 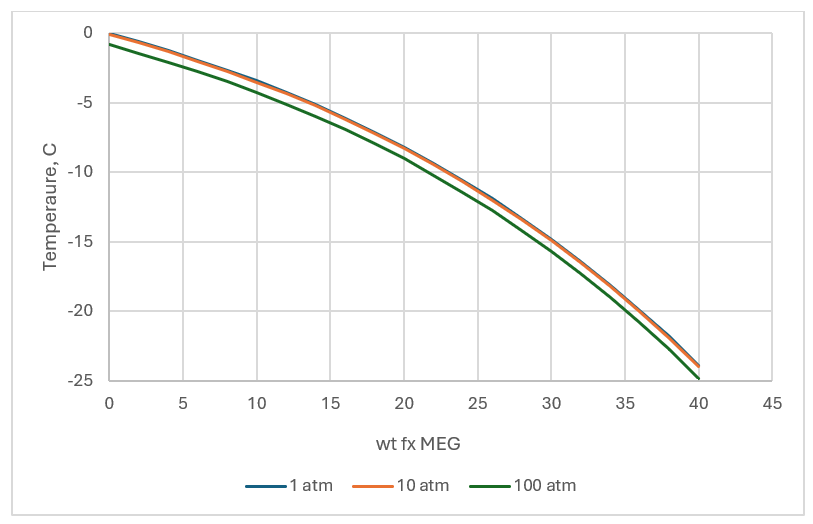
The same “colligative” property applies when separating H2O and MEG via distillation. As the MEG fraction increases in the mixture, the boiling temperature increases. The plot above and below also shows the effects of total pressure on the curves. While pressure does not affect the freezing point, it has a significant effect on the boiling point. The latter is a property we can use when designing the separation process.
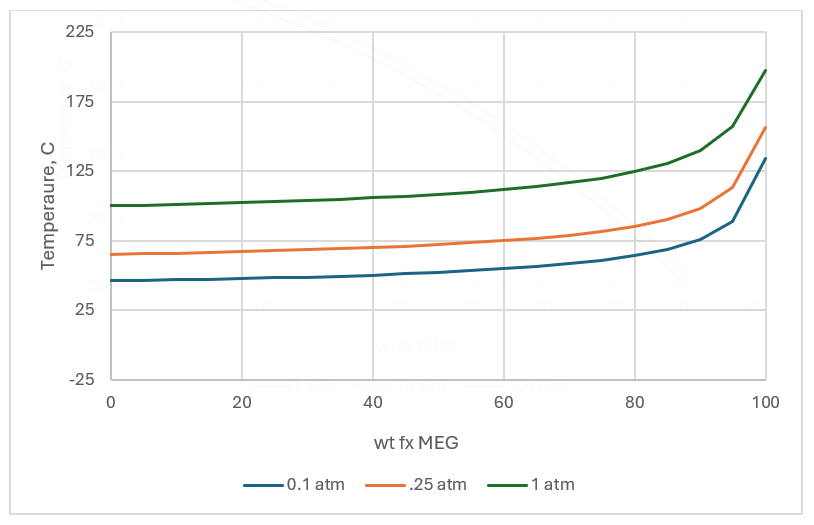
If the produced fluid contains just light gases, water, and MEG, then regeneration would be a cinch. This, however, is not the case. The fluid can also include a brine that contains dissolved salts. This complicates both the injection (inhibition) and regeneration process because salts are soluble in MEG. They are less soluble than in H2O, and that has a two-fold effect. If the regenerated MEG is saturated with salt, then reinjecting MEG induce mineral scaling in the flowline. This is not the focus of the current blog, and so we will move to the next complication. Regenerating MEG usually means evaporating the water. This leaves behind the original salts and the MEG. Since the salts are less soluble in MEG, they tend to drop out.
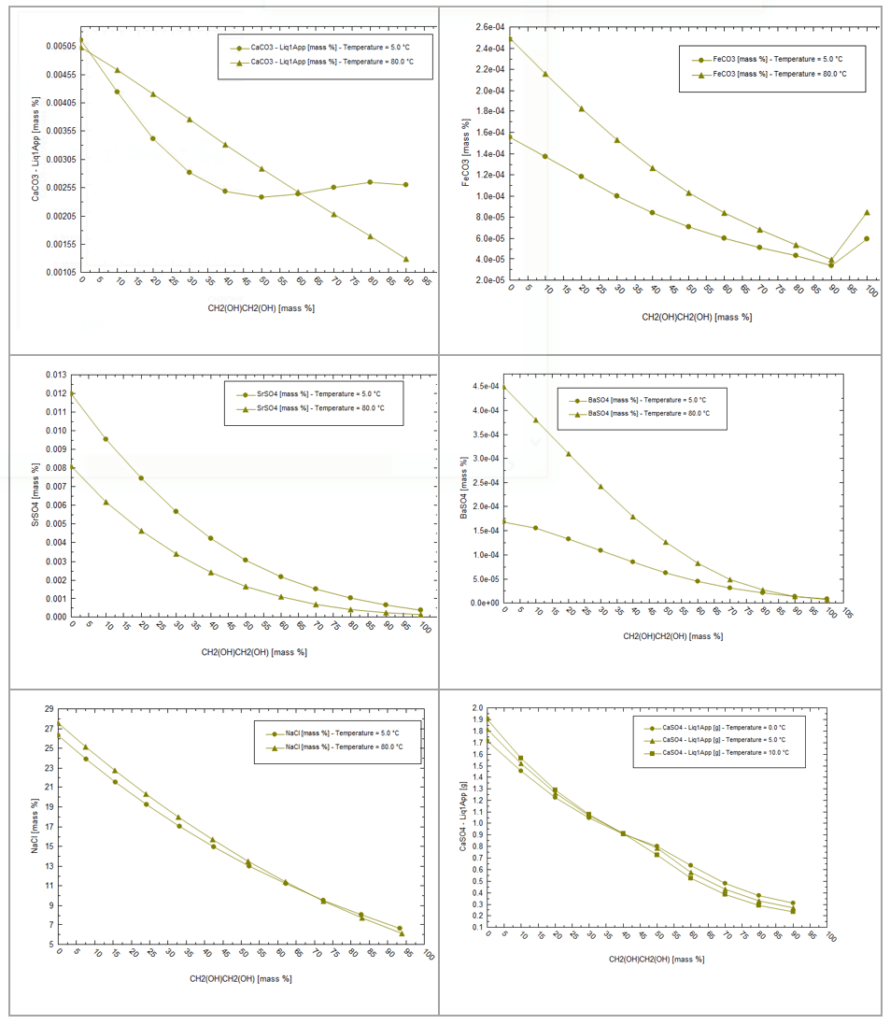
Below are some solubilities of common scales in H2O and MEG. The far left is salt solubility in H2O and the far right is solubility in MEG. The plot has two temperatures, 5C for MEG injection and 80C for MEG regeneration. In nearly all mixtures, salt solubility decreases as MEG increases, and in some cases their solubility differs by an order of magnitude. This will come into play when we discuss the regeneration step.
Example situation – regenerating MEG in a fluid containing dissolved salts
We will use as an example case a produced brine containing 45wt% MEG, 69,000 ppm Na+Cl, 5700 ppm Ca/Mg hardness (as CaCO3), and other ions (Clients can ask for the OLI Studio file if curious). One conceptual design is to evaporate the water from brine and then remove the salts from the MEG. A second approach is to evaporate the H2O and MEG and leave the salts. The water and MEG can then be separated from each other using their boiling point differences. Both approaches require that we know the properties of the mixture as it is heated and evaporated. Since we are focusing on the fluid properties, there is no engineering equipment to describe. So, the calculations shown below are based on what can occur as we evaporate the fluid under different conditions.
Separating water from the MEG-salt
The first step is to evaluate the properties as 1 kg of brine evaporates at three pressures, 0.1, 0.25, and 1 atm. Pressure affects the evaporation temperature which in turn affects other brine properties like salt solubility and MEG evaporation.
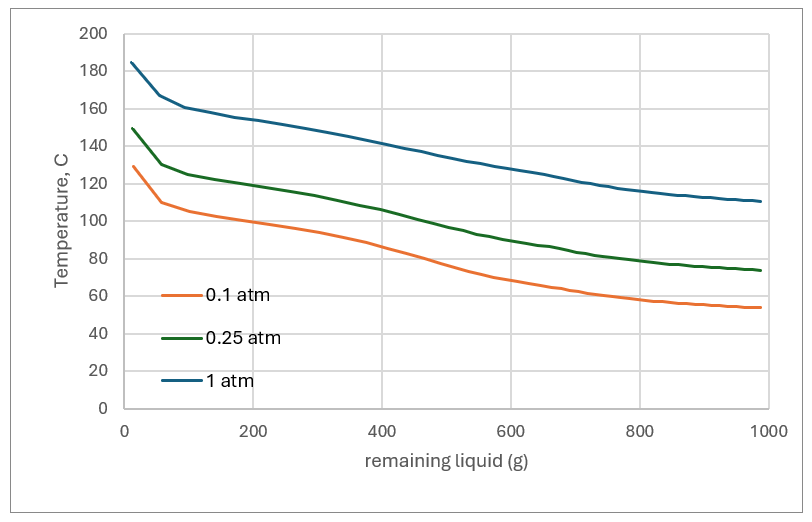
The first plot shows the MEG:H2O mass ratio in the remaining liquid as evaporation proceeds. The far right of the plot is the original 1 kg of liquid where the MEG:H2O ratio is ~1:1. As the liquid evaporates (moving from right to left) the MEG:H2O ratio in the remaining liquid increases because H2O is more volatile than MEG. If the operator’s target is to regenerate the MEG to 80% MEG (a 4:1 MEG:H2O ratio), then the liquid needs to be evaporated down to 550 grams at 1atm and 600 grams at 0.1 atm.
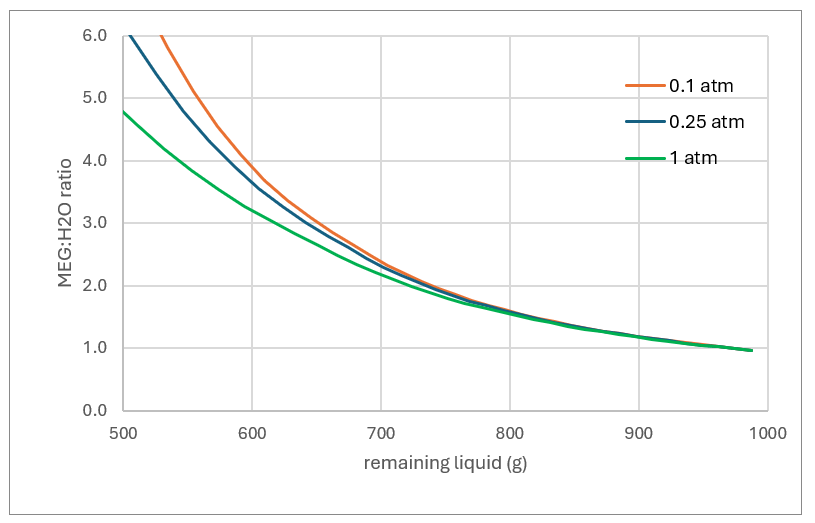
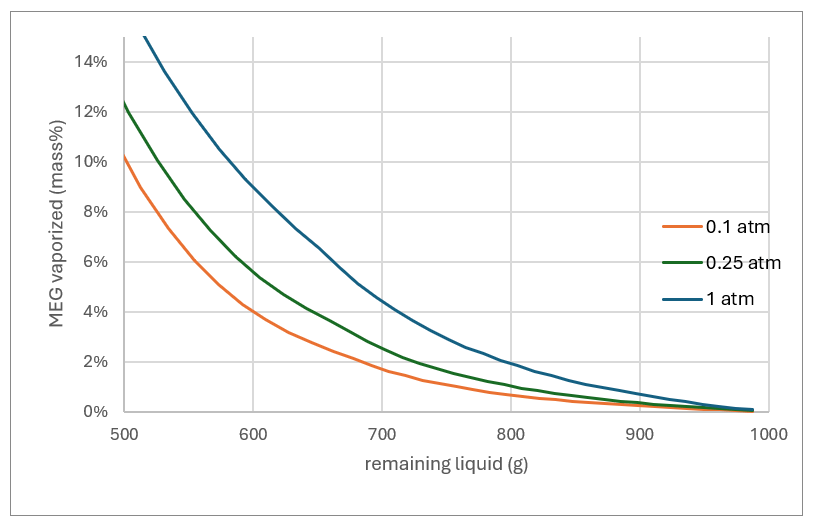
Higher pressures mean higher temperatures, and at 1 atm, more of the MEG evaporates. Using the same target of a 4:1 MEG:H2O mass ratio, about 12% of the MEG evaporate with the water. By comparison, if evaporation is run under vacuum, 0.1 atm, only about 4% of the MEG evaporates for the same MEG:H2O ratio. So, lower pressures means less MEG loss.
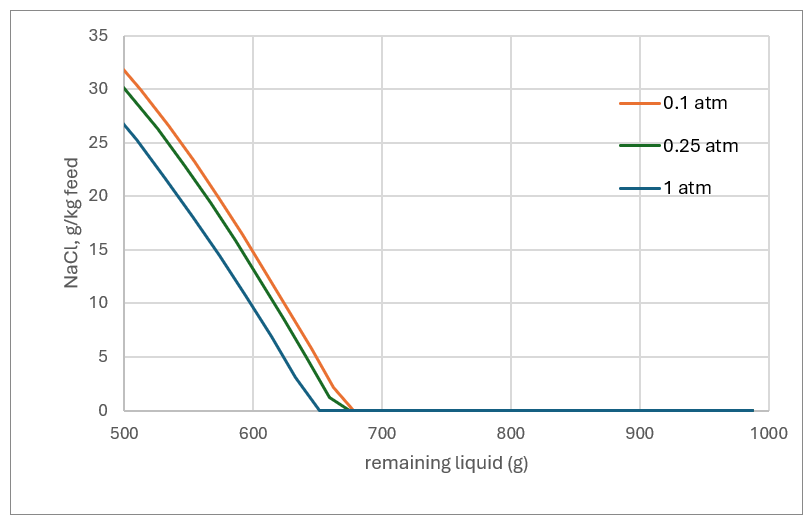
Eventually the remaining liquid becomes saturated with the dissolved salts. This is for two reason. First, there is less solvent for the salts to dissolve into. Second, the liquid becomes MEG-enriched and the salts are less soluble in that solvent. NaCl is predicted to drop out of the liquid before evaporation reaches the 4:1 mass ratio, regardless of pressure. For the 1 atm case (550 g remaining liquid) the amount of salt produced is 20 grams per kg of starting liquid. for the 0.1 atm case (600 g remaining liquid), about 15 grams of salt forms per kg of starting liquid. The temperature difference between the low and high pressure operation plays a factor. Higher pressure means a higher liquid temperature, and salts are generally more soluble at higher temperatures. So, there are two competing properties that produce these results, the lower solubility in MEG and the higher soubility at the higher temperatures. In either case, some form of solids handling is needed since salt will form if we evaporate to this extent.
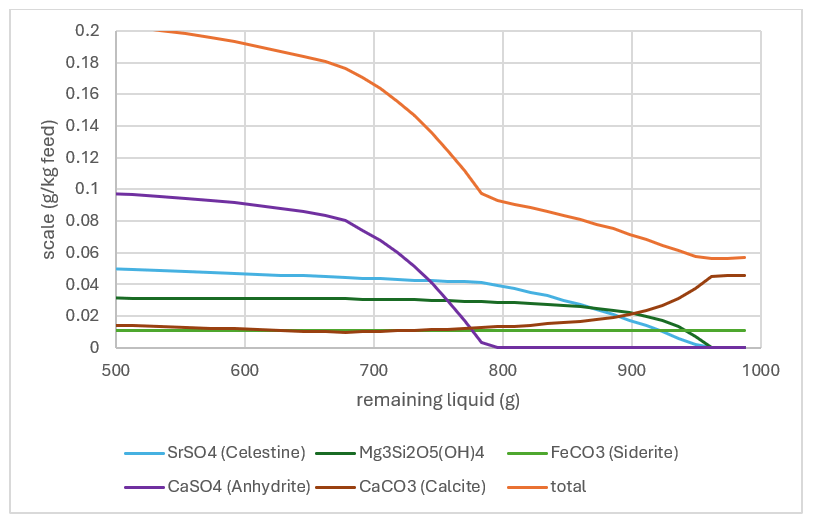
Salt (NaCl), is not the only scale that can form. Other, more adherent deposits are predicted in this example. Below is a plot of five potential scales. The amount that forms is up to 0.2g per kg feed, or about 200 ppm. These more adherent scales require attention so that they do not foul heat exchangers and other key process units.
Evaporating MEG and H2O, leaving the salts
A conceptual design could be to evaporate the MEG and water, leaving the salts. This would be consistent with an evaporator-crystallizer process; something that can manage high solids slurries. Instead of targeting a MEG:H2O ratio, the target is maximum MEG evaporation. The same study plots are used to understand how the process will operate, but the focus instead is the far left of the plot where the liquid mass approaches zero.
As MEG and H2O evaporate, the liquid boiling point increases. If the liquid is evaporated at atmospheric conditions, the temperature approaches 180 C – MEG degradation is now a factor. At 0.1 atm conditions, the temperature stays below 130C. Thus, there is a balance between the lower cost atmospheric evaporation and the cost of added heat and degrading the MEG.
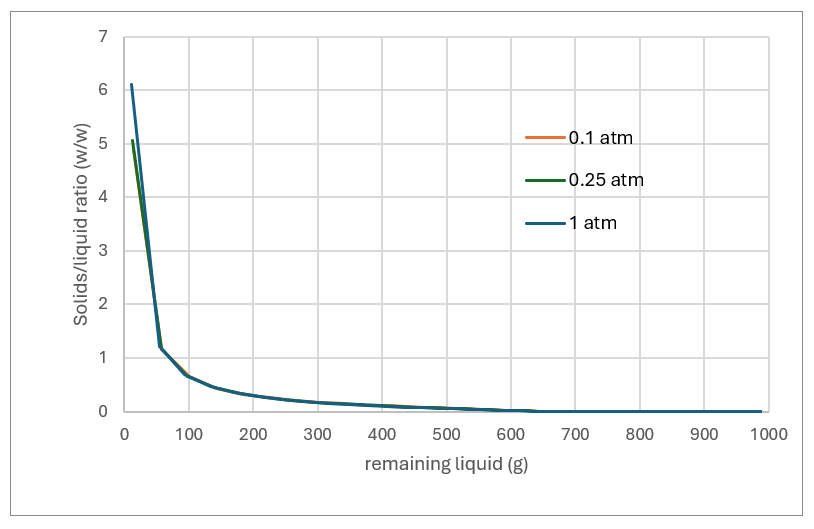
Evaporating the solvent means leaving behind a solids slurry. As the MEG-H2O evaporates, the slurry becomes solids-ladened. Nearly all of it is NaCl along with the scaling solids shown above. By about 95% the solids-liquid mass ratio is 3:1. Designing for this is where the evaporator-crystallizer manufacturers have expertise and they can help the operator determine the extent of evaporation.
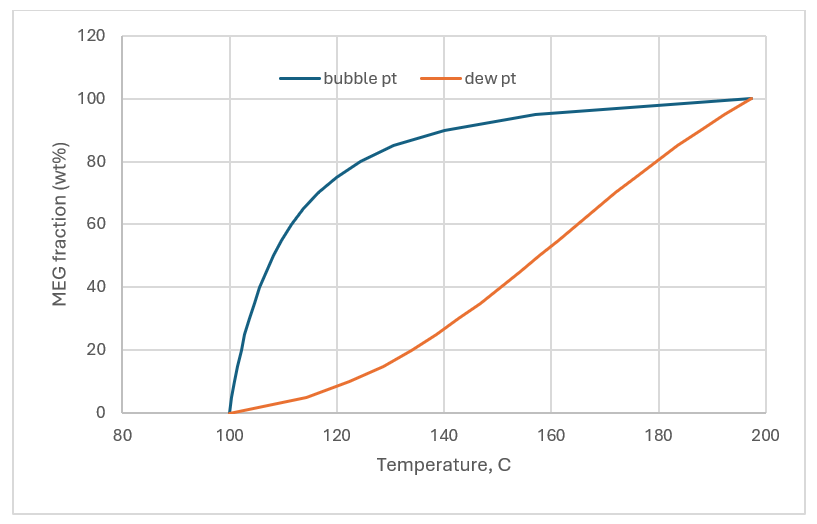
Once the MEG and H2O are in the vapor phase, they can be separated using their boiling point differences. The plot below show how the MEG separates in the vapor and liquid as a function of process temperature. At 123C for example, the condensing liquid is 80 wt% MEG, the purity target. This type of separation is common in chemical engineering, and so most readers will know this type of separation already.
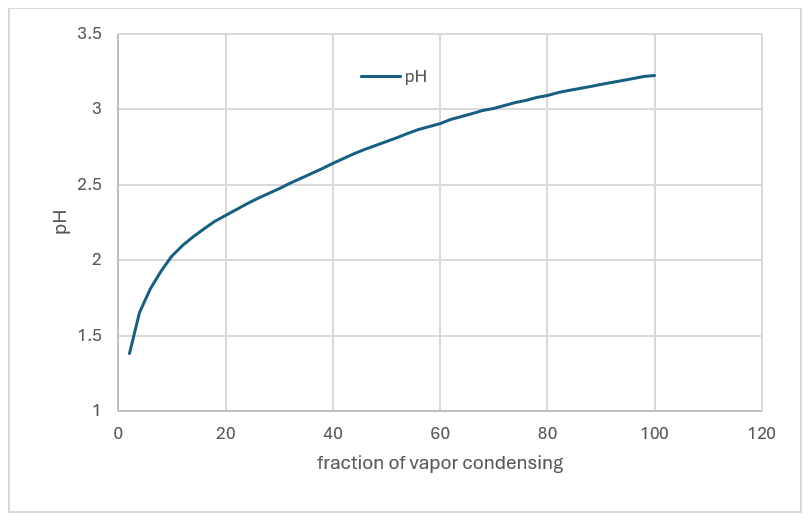
What is interesting though is that evaporating the MEG-H2O also causes volatile acids eo evaporate. If we take the condition where 95% of the MEG is evaporated at 0.1 atm, the vapor will contain ~10 ppmV HCl and 60ppmV CO2. Consequently, as the vapor condenses, the liquid produced will be acidic. The plot below shows the pH of the condensed liquid as the MEG-H2O vapor condenses. The initial liquid is a most with a pH of 1.3. As condensation increases, the acid is diluted, and the pH increases to 3.1 – still corrosive to carbon steel. Thus, a new factor needs to be considered, general corrosion of the condensing equipment.
Summary
Recovering MEG is critical to cost-effective deepwater natural gas production. Recovery, however, is complicated when the returning liquid contains dissolved solids. Knowing how the fluid property behaves during MEG recovery is key to designing the right process. Our goal in this blog was to show how properties like boiling point rise, pressure, and solid solubility affect the fluid properties. We hope that this information helps engineers to design and optimize MEG regeneration units specific to their production conditions.
To discuss this topic in more detail please contact us.