End-of-life lithium-ion batteries as secondary sources of critical materials
Lithium-ion batteries (LIBs) have been widely used in portable electronic equipment and electric vehicles because of their high energy density, low self-discharge rate, and light weight. With the large consumption and growing demand for LIBs (see Figure 1), a large number of spent LIBs will be generated due to their limited average life. Disposal of the spent LIBs causes environmental pollution because they contain heavy metals, organic chemicals, and plastics among other harmful materials. At the same time, they have been identified as secondary resources of critical metals such as nickel, cobalt, manganese and lithium (i.e., Ni, Co, Mn, Li). Therefore, recycling spent LIBs is necessary for protecting the environment as well as saving resources.
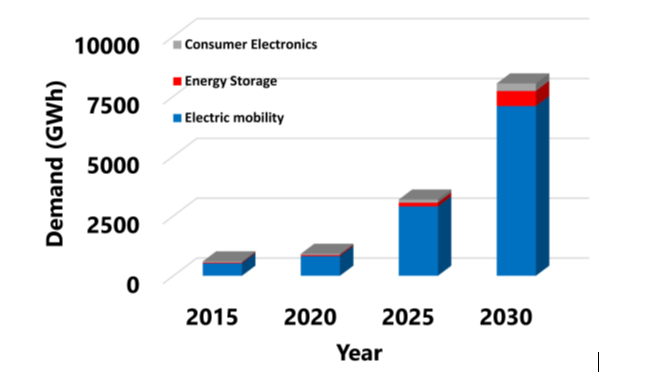
Figure 1. Global demand for battery technologies by application from 2015 to 2030 (Tawonezvi et al. (2023))
Metals from lithium-ion batteries can be recovered through a variety of processes such as pyrometallurgy, hydrometallurgy, biometallurgy, leaching, solvent extraction and ion exchange. All these processes have advantages and disadvantages. For example, pyrometallurgy creates harmful gases and waste slag whereas hydrometallurgy requires various acids and solvents, which can create toxic waste. Conversely, biometallurgy is an environmentally friendly process but may not be cost-effective. To make LIBs recycling environmentally friendly and economically feasible, isolation of high-purity critical materials must be performed with a minimum number of steps and should minimize chemical waste.
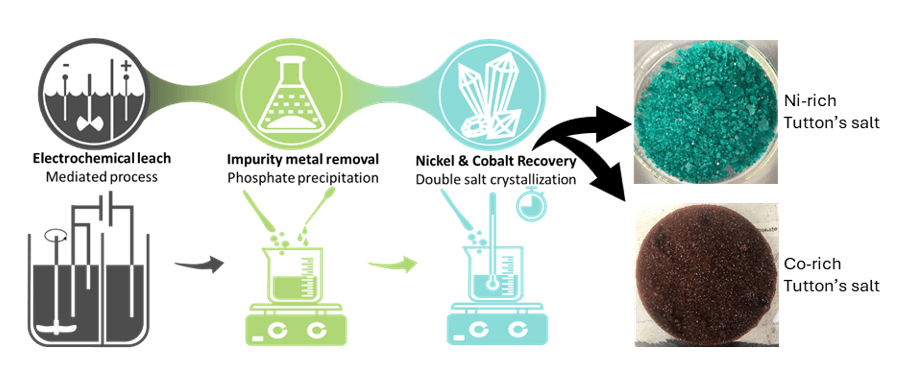
To recover valuable metals from end-of-life LIBs, they have to be converted into a black mass, which usually contains critical cathode materials (Ni, Co, Li, Mn) and impurities such as iron, aluminum, and copper (Fe, Al, Cu). Modeling chemistry and phase equilibria in these systems makes it possible to accelerate process design by reducing the necessary number of steps in processing LIBs black mass. Recently, just three steps were used in processing black mass, i.e., electrochemical leaching, precipitation of insoluble phosphates and fractional precipitation. In particular, Diaz et al. (2020) used electrochemical leaching of a black mass and plated out Cu on the cathode. Then, the sparingly soluble phosphates of Al and Fe were removed using ammonium phosphate as reported by Klaehn et al. (2023). In the last step, Klaehn et al. (2024) developed a fractional precipitation process to recover Ni and Co as well as Mn.
Experimental data and modeling using the Mixed-Solvent Electrolyte (MSE) framework in ternary NiSO4-(NH4)2SO4-H2O and CoSO4-(NH4)2SO4-H2O systems
To establish conditions for recovering Ni and Co among other metals, experimental data on solubilities and phase equilibria in aqueous cobalt, nickel and ammonium sulfate systems, CoSO4-NiSO4-(NH4)2SO4-H2O, can be very beneficial. Symbols in Figures 2 and 3 show solid-liquid equilibria in ternary NiSO4-(NH4)2SO4-H2O and CoSO4-(NH4)2SO4-H2O systems, respectively. It turns out that both cations (i.e., Ni2+, Co2+) form double sulfate salts with ammonium cations, the so-called Tutton’s salts.* In both cases, the dominant crystalline form of the double salt is a hexahydrate (see Figures 2 and 3). However, the solubilities of these double salts are considerably different. Figure 2 shows the solubility of (NH4)2Ni(SO4)2∙6H2O at 25oC as a function of two constituent salts, (NH4)2SO4 and NiSO4, solubilities of which are also shown.
*Tutton’s salts: MeII(NH4)2(SO4)2∙6H2O
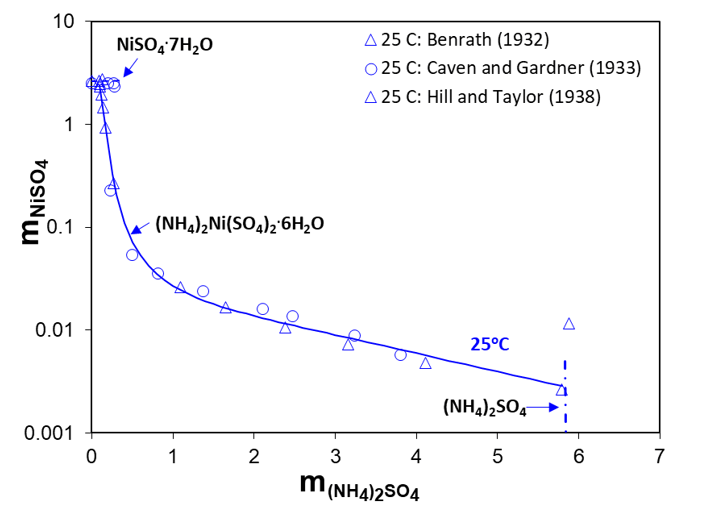
Figure 2. Solid-liquid equilibria in the ternary system NiSO4–(NH4)2SO4–H2O at 25°C. The lines are calculated with the MSE model
The solubility of (NH4)2Co(SO4)2∙6H2O at 0, 25 and 50oC as well as its two constituent salts, (NH4)2SO4 and CoSO4, are shown in Figure 3. At 25oC, the solubilities of both double sulfate salts decrease with the addition of ammonium sulfate, but they are very different. The addition of ammonium sulfate can reduce the concentration of Ni in solution by as much as three orders of magnitude (see Figure 2). On the other hand, Figure 3 shows that the concentration of Co drops only about two orders of magnitude when ammonium sulfate is added. However, in both cases the addition of ammonium sulfate is a highly effective way to precipitating nickel or, to a lesser extent, cobalt from solution.
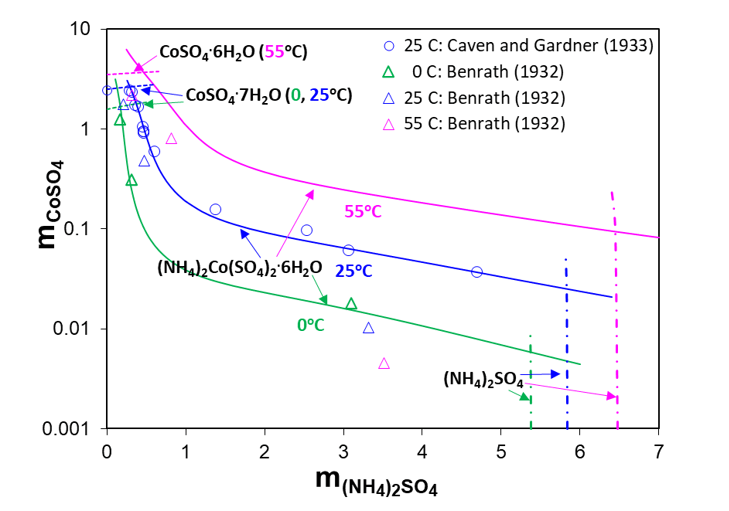
Figure 3. Solid-liquid equilibria in the ternary system CoSO4–(NH4)2SO4–H2O at fixed temperatures (0°C, 25°C, and 50°C)
Figure 4 presents experimental solubilities of Ni ammonium sulfate double salt at temperatures from 0 to 70oC. The solubilities of Co double salt as a function of temperature are illustrated in Figure 5. To develop and extend the conditions for recovering Ni and Co, a thermodynamic model was constructed using the Mixed-Solvent Electrolyte (MSE) framework. The MSE model is parameterized using a bottom-up approach, i.e., by developing model parameters for constituent simple subsystems (primarily binary mixtures) followed by more complex ternary and higher-order systems. The parameters of the MSE model were previously developed to accurately reproduce solubilities in the binary systems CoSO4-H2O and NiSO4-H2O. In the study of Klaehn et al. (2024), the MSE model was extended to ternary mixtures of NiSO4 and CoSO4 with (NH4)2SO4. The MSE parameters were developed based on experimental solubilities in these ternary systems presented in Figures 2-5. Subsequently, the model was validated by a comparison of calculated (solid lines) and experimental (symbols) data of solubilities of Ni and Co sulfates in the presence of ammonium sulfate or Ni and Co double salts as functions of temperature, which are generally in good agreement. The calculated solubilities of both Ni and Co double salts increase between 0 and 100oC (see Figures 4, 5) but the solubility of (NH4)2Ni(SO4)2∙6H2O is lower than that of (NH4)2Co(SO4)2∙6H2O. This is valuable information for separating Ni from Co by varying the temperature.
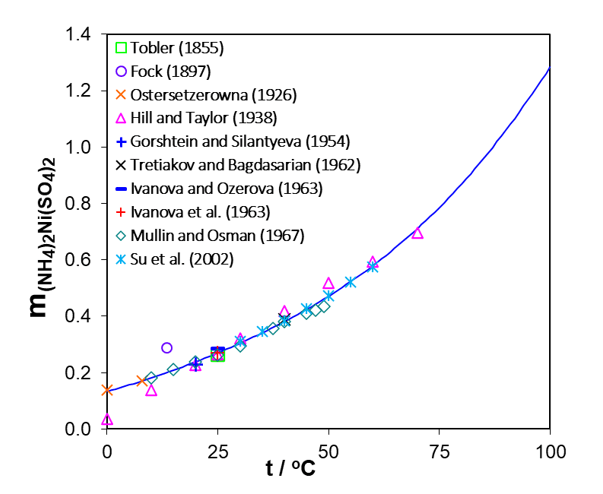
Figure 4. Temperature dependence of the solubility of the double salt (NH4)2Ni(SO4)2·6H2O
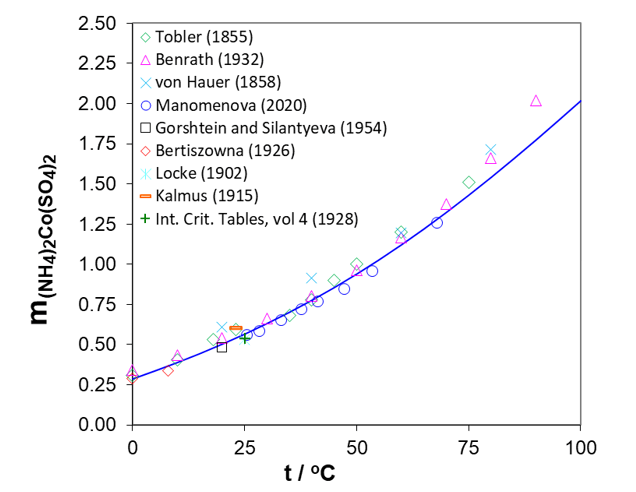
Figure 5. Temperature dependence of the solubility of the double salt (NH4)2Co(SO4)2·6H2O
Application of the MSE model to multiple cation/anion mixtures containing both Ni and Co
Due to their structural similarity, the cobalt and nickel ammonium double salts can form a solid solution in a full range of composition. In general, the solid solution can be represented as (NH4)2CoxNi1-x(SO4)2∙6H2O, where the mole fraction, x, ranges from 0 to 1. Surprisingly, this statement was confirmed by Gorshtein and Silant’yeva (1954) who investigated the solubility of the solid solution mentioned above in the full concentration range. In fact, Klaehn et al. (2024) discovered the precipitation of a high purity solid solution (NH4)2Co0.22Ni0.78(SO4)2·6H2O during their fractional crystallization. Figure 6 shows the relationship between the composition of the solid solution (expressed as weight % of cobalt relative to the total mass of the metal, Co + Ni, in the solid phase) and the concentrations of both cobalt (blue curve) and nickel (purple curve) in the aqueous solution. It is notable that the relationship between the compositions in the liquid and solid phases is practically linear for Co and nearly linear for Ni. This indicates that the solid solution is reasonably close to ideal with respect to the two end members, i.e., (NH4)2Ni(SO4)2·6H2O and (NH4)2Co(SO4)2·6H2O. The composition of the solid solution (NH4)2Co0.22Ni0.78(SO4)2·6H2O is denoted by the dotted lines. Based on the measurements of Gorshtein and Silant’yeva (1954), it was possible to develop thermochemical properties of this solid solutions using the MSE model.
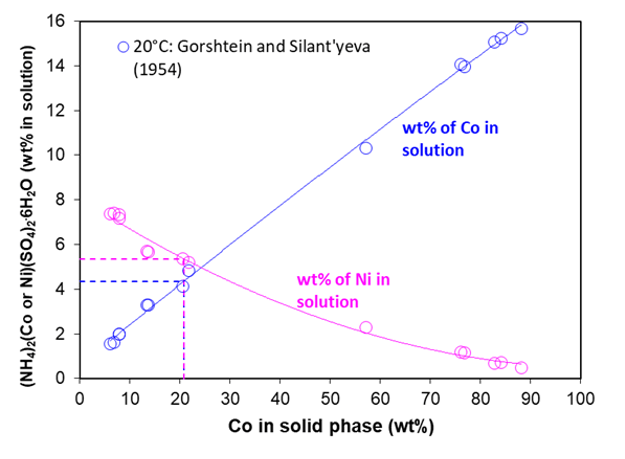
Figure 6. Solubility of (NH4)2CoxNi1-x(SO4)2·6H2O solid solutions at 25°C: Relationship between the concentration of cobalt and nickel in solution as a function of the composition of the solid phase (expressed as weight % of the metal excluding other elements). The dotted lines indicate the concentrations for the solid solution identified in this study: (NH4)2Co0.22Ni0.78(SO4)2·6H2O
The thermodynamic analysis described here indicates that nickel and cobalt can be effectively precipitated from sulfate solutions in the form of hydrated double sulfate salts by adding ammonium sulfate. Since the Ni and Co double salts form a solid solution in the full range of composition, the addition of ammonium sulfate to a mixed nickel – cobalt aqueous system will result in the precipitation of a solid solution. Assuming that these solid solutions are close to ideal, their standard-state properties can be calculated based on the MSE model. For more information or to talk to our experts contact us.
References:
Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. “Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis,” Energies 2023, 16, 1365. https://doi.org/10.3390/en16031365
Diaz, L.A.; Strauss, M.L.; Adhikari, B.; Klaehn, J.R.; McNally, J.S. and Lister, T.E. “Electrochemical-assisted leaching of active materials from lithium ion batteries,” Resources, Conservation and Recycling, 2020, 161, 104900.
Klaehn, J.R.; Shi, M.; Diaz, L.A.; Molina, D.E.; Reich, S.M.; Palasyuk, O.; Repukaiti, R. and Lister, T.E. “Removal of impurity Metals as Phosphates from Lithium-ion Battery Leachates,” Hydrometallurgy, 2023, 217, 106041.
Klaehn, J.R.; Shi, M.; Diaz, L.A.; Molina, D.E.; Repukaiti, R.; Madani Sani, F.; Lencka, M.; Anderko, A.; Arulsamy, N. and Lister, T.E. “Fractional precipitation of Ni and Co double salts from lithium-ion battery leachates,” RSC Sustainability, 2024. DOI: 10.1039/D4SU00303A https://pubs.rsc.org/en/content/articlelanding/2024/su/d4su00303a
Gorshtein, G.I. and Silantyeva, N.I. “Distribution of isomorphic and iso-dimorphic components between solid and liquid phases during crystallization from aqueous solutions,” Zhurnal Obshchei Khimii 1954, 24, 29-36.