Flow-accelerated corrosion (FAC) is recognized as the primary cause of failure in carbon steel equipment, particularly in facilities where internal piping is exposed to turbulent, high-velocity flows of only water phase (single-phase FAC) or water-steam phase (two-phase FAC).1–3 Examples where flow induced corrosion can occur include boilers that are used for hydrocarbon recovery from oil sands, water/steam cycle boilers, power plants, boiler feedwater systems, heat recovery steam generators, steam generator blowdown systems, heater drain lines, flashing lines to the condenser, etc. (FAC does not occur in dry steam).1,2,4 FAC can cause steam/water leaks, compromising both equipment and personnel safety. Under FAC conditions, turbulent flow or high-water flow velocity can increase the mass transfer of ionic species of iron at the oxide surface and accelerating the corrosion rate.5 Figure 1 shows two examples of FAC damage in the form of severe V-shaped deterioration regions below the injection point and a wavy pattern corrosion in a tube connecting to the high-pressure economizer header.
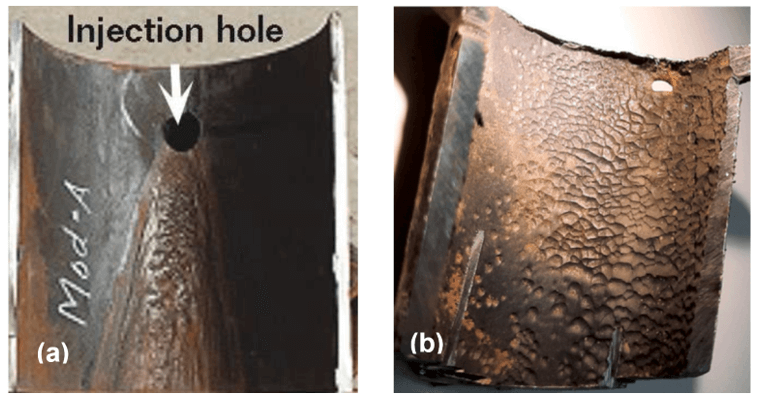
Figure 1. (a) V-shape FAC attack close to the injection point (picture from Ref. [5]), and (b) wavy pattern FAC in the tube connecting to the high-pressure economizer header (picture from Ref. [6])
Under high-temperature, alkaline, anaerobic conditions, a thin, stable layer of iron oxide (mostly magnetite, Fe3O4) forms in the temperature range of 100–300°C, which is the typical temperature range in which FAC occurs.4, 7 Magnetite is protective but can dissolve under harsh FAC conditions, influenced by factors such as temperature, pressure, pH, fluid chemistry, salinity, and piping materials.8 Understanding magnetite solubility and its stability region is becoming increasingly important in corrosion modeling studies, particularly with the continued development of offshore production in deepwater wells operating under High Temperature–High Pressure (HTHP) conditions.8
Given the importance of predicting FAC incidents under HTHP, Peiming Wang, OLI’s retired expert in electrolyte thermodynamic modeling, established the foundational framework in OLI’s Mixed Solvent Electrolyte (MSE) V12 database and provided essential conceptual insights into the modeling principles of solubility for different iron oxide/hydroxides (e.g., Fe3O4, Fe2O3, FeOOH, Fe(OH)3, etc.) under HTHP conditions. Measuring magnetite solubility, the formation constants of Fe-containing ligands, and the analytical detection of dissolved Fe complexes (especially Fe(III)) during iron oxide dissolution under HTHP FAC conditions is extremely difficult. For magnetite, this is even more complicated than for other iron oxides due to the reductive dissolution potential from Fe(III) to Fe(II).9 This is where advanced modeling and predictive techniques are needed to forecast the solubility and stability of iron oxides and prevent HTHP FAC. OLI’s MSE model is capable of precisely predicting the partitioning of iron among its principal chemical forms, i.e., Fe(II) and Fe(III) species, and evaluating the total solubility of iron oxides under HTHP FAC conditions, highlighting MSE’s unique capabilities in speciation calculations. This can provide critical insight for industry practitioners and specialists in material integrity to assess and model corrosion, enabling risk prediction for facilities operating under HTHP. Figure 2a shows an example of the model predictions for magnetite solubility in feed water saturated with hydrogen overpressures at 1 atm at 300 °C. The contribution of different Fe(II,III) species to total solubility of magnetite under this condition is shown in Figure 2b.
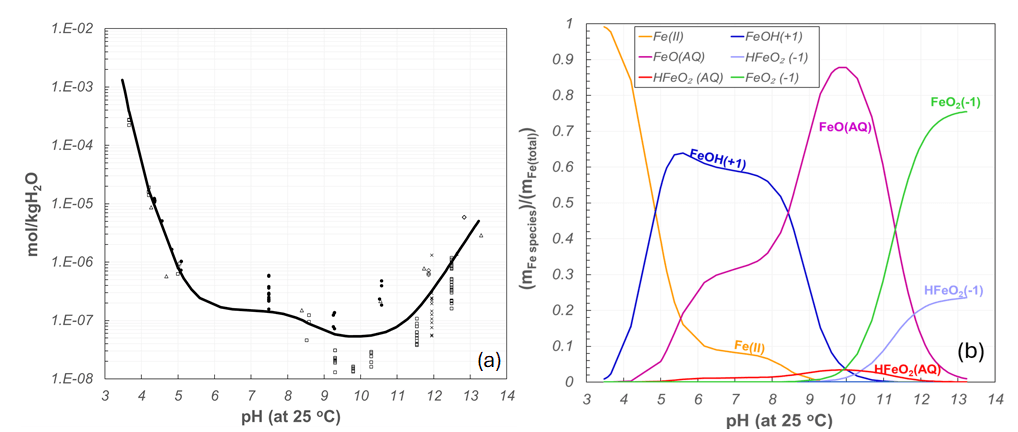
Figure 2. MSE model (solid line) for magnetite solubility at 300 °C, where the symbols represent data from Ref. [10–12] and (b) MSE predictions of the concentration of dominant iron oxide/hydroxide species among its different chemical forms resulting from the dissolution of magnetite at 300 °C
As shown in Figure 2b, under such redox conditions at 300 °C, MSE predicts ferrous oxide/hydroxide species (Fe2+, FeOH+, FeOaq.) as the dominant products of magnetite dissolution at pH levels lower than 11. However, at pH higher than 11, magnetite dissolves into the solution in the form of mostly ferric oxide/hydroxide ions (FeO2–), with a minor quantity of ferrous oxyhydroxide species (HFeO2–)). Different positively charged iron complexes produce different hydrolysis products and can engage in specific complexation processes with various ligands (e.g., organic acid ligands, acetate, carboxylic acids, etc.), making the oxide layer either soluble or stable (depending on the tendency factor for oxide formation).13, 14 On the other hand, negatively charged iron species contribute to specific surface complexation and adsorption mechanisms involved during iron dissolution or oxide film degradation.15 OLI’s V12 offers an extensive database built based on highly accurate thermodynamic data and equilibrium constants for all these types of reactions between different iron species and ligands, complexation reactions of iron ions, cation hydrolysis, anion adsorption, etc.
Hydrothermal brines in geothermal power plants typically contain various concentrations of ions such as bicarbonate, carbonate, borate, and chloride.16, 17 These anions exhibit various degrees of affinity for complexation with ferrous and ferric species, leading to increased solubility of iron oxides. For example, chloride can form ferrous chloride, hydroxy-chloride, and higher-order chloride complexes, which may be thermodynamically more stable than magnetite, increasing its solubility under HTHP conditions.17 OLI’s V12 first-principles corrosion prediction tool models these complexations and their impact on iron oxide stability for a wide range of anion chemistries. For example, Figure 3 shows the impact of chloride on the stability diagram of iron, generated using OLI V12’s Corrosion Analyzer. The diagram maps the possible stable iron species in boiler blowdown water at 300 °C and 110 atm.
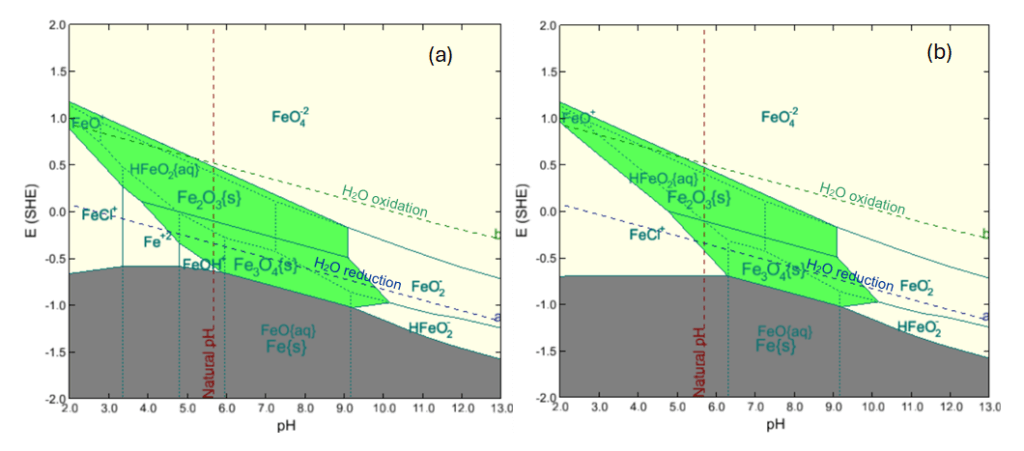
Figure 3. Stability diagrams created by OLI V12’s Corrosion Analyzer for iron in a boiler blowdown water at 300 °C and 110 atm in (a) absence of chloride ion and (b) presence of 2050 ppm chloride ion
A comparison of Figures 3a and 3b shows that the presence of chloride expands the region of dissolved iron complexes (white regions) while reducing the stability regions of iron oxides (green regions). The Pourbaix diagram shows that in alkaline environments, Fe(III) species, such as FeO2–, dominate, while in acidic conditions (pH < 6), Fe(II) species are predominantly the stable ions in the aqueous phase. Ferrate species (FeO42-) is highly unstable, forming only under extremely alkaline and oxidizing conditions, with a strong tendency to reduce to lower oxidation state iron complexes. Under HTHP conditions, chloride ions can facilitate the dissolution of iron oxides through complexation and formation of soluble chloride-containing iron species (according to the following reactions), which destabilize the solid iron oxide phase. These processes shift the equilibrium away from the stability of magnetite/hematite, enhancing their solubility in aqueous environments:18
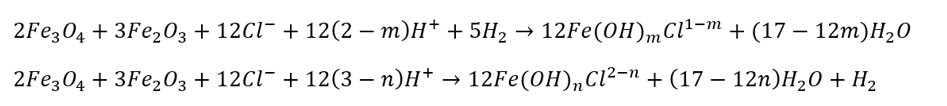
Chloride can increase the solubility of magnetite by facilitating the formation of chloro- or hydroxyl-chloro complexes, which exhibit greater thermodynamic stability compared to magnetite, thereby shifting the equilibrium towards magnetite dissolution.19
In addition to inorganic ions such as chloride, systems operating under HPHT conditions, including steam injection, boiler feed water, boiler blowdowns, and enhanced oil recovery process water, may also contain various dissolved organic materials. These organic impurities with polar functional groups can also affect the stability of iron oxide passive films under steam-generating HTHP, directly impacting FAC. Figure 4 lists some of the organic components existing in OLI V12 that can potentially affect HTHP FAC of carbon steel. This topic will be further discussed in a future blog.
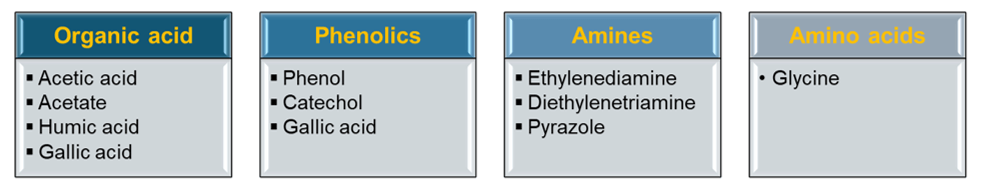
Figure 4. Some of organic components existing in OLI V12 that can potentially affect HTHP FAC of carbon steel (iron-organic complexation information included in V12 database)
OLI’s V12, with a database of more than 380 Fe-containing ions, complexes, solids, oxides, etc., along with precise thermodynamic parameters for their interactions in various aqueous or non-aqueous solvents, is capable of simulating the impact of inorganic/organic compounds on the stability and solubility of iron oxides under HTHP FAC conditions. It provides accurate predictions of iron species partitioning across different phases, showcasing MSE’s unique capabilities in speciation calculations related to high-temperature carbon steel corrosion issues under FAC. To learn more and share ideas on this topic, contact us.
References
[1] S. Findlan, “Recommendations for an effective flow-accelerated corrosion program (NSAC-202L-R3)”, EPRI Report TR-1015425, Palo Alto, CA, 2007.
[2] B. Chexal, “Flow accelerated corrosion in power plants”, EPRI Report TR-106611- R1, Palo Alto, CA, 1998
[3] B. Dooley, D. Lister, “Flow-accelerated corrosion in steam generating plants”, Power Plant Chemistry 20 (2018) p. 194.
[4] M. Vepsäläinen, T. Saario, “Magnetite dissolution and deposition in NPP secondary circuit”, Research Report VTT-R-09735-10, 2010.
[5] M.H. El-Sayed, “Flow accelerated corrosion in gas compression piping”, Materials Performance, NACE International 54 (2015) p. 46.
[6] P. Khunphakdee, B. Chalermsinsuwan, “Review of flow accelerated corrosion mechanism, numerical analysis, and control measures”, Chemical Engineering Research and Design 197 (2023) p. 519.
[7] E.M. Pavageau, R. Michel, “Influence of chemistry and temperature on the FAC rate of carbon steel: an example of study done at EDF R&D thank to CRIOCO loop”, International FAC Conference on Flow Accelerated Corrosion, Lyon, France 2008.
[8] C. Yan, P. Guraieb, J. Huang, E. Contreras, R.C. Tomson, “Solubility study of magnetite under extreme high pressure and high temperature”, Rice University, OTC-25216-MS, 2014.
[9] S.E. Ziemniak, M.E. Jones, K.S. Combs, “Magnetite solubility and phase stability in alkaline media at elevated temperatures”, Journal of Solution Chemistry 24 (1995) p. 837.
[10] P.R. Tremaine, J.C. LeBlanc, “The solubility of magnetite and the hydrolysis and oxidation of Fe2+ in water to 300 °C”, Journal of Solution Chemistry 9 (1980) p. 415.
[11] F. H. Sweeton, C. F. Baes, JR., “The solubility of magnetite and hydrolysis of ferrous ion in aqueous solutions at elevated temperatures”, J. Chem. Thermodynamics 2 (1970) p. 479.
[12] J.Y. Chung, K.J. Lee, “The solubility of magnetite and nickel ferrite in high temperature aqueous solutions”, High Temperature Science 30 (1990) p. 51.
[13] E.L. Shock, C.M. Koretsk, “Metal-organic complexes in geochemical processes: Calculation of standard partial molal thermodynamic properties of aqueous acetate complexes at high pressures and temperatures”, Geochimica et Cosmochimica Acta 57 (1993) p. 4899.
[14] G. K. Johnson, J.E. Bauman, JR., “Equilibrium constants for the aquated iron(II) cation”, Inorganic Chemistry 17 (1978) p. 2775.
[15] P.C. Torres, R.P. Nogueira, V. Fairen, “Forecasting interface roughness from kinetic parameters of corrosion mechanisms”, Journal of the Electrochemical Society 529 (2022) p. 109.
[16] R.H. Byrne, D.R. Kester, “Solubility of hydrous ferric oxide and iron speciation in seawater”, Marine Chemistry 4 (1976) p. 255.
[17] D.A. Palmer, K.E. Hyde, “An experimental determination of ferrous chloride and acetate complexation in aqueous solutions to 300°C”, Geochimica et Cosmochimica Acta 57 (1993) p. 1393
[18] Y. Zeng, R. AI, F Wang, “Solubility of the magnetite + hematite buffer assemblage and iron speciation in sodium chloride solutions at 300 °C and 500 bars”, Geochimica et Cosmochimica Acta 53 (1989) p.1875.
[19] S. Wang, H.P. EUGSTER, G. Wilson, “Solubility of magnetite and iron speciation in supercritical HCl and NaCl solutions”, Geol. Soc. Amer. Abstr. Prog. 16 (1984) p. 686.