Mineral scaling is a significant challenge in HPHT environments in the Upstream Oil and Gas industry
Mineral scaling is a serious problem that has a high impact on operation costs in the oil and gas production industry. Formation of scales can hinder fluid flow leading to low operational efficiencies, cause equipment damage, conceal corrosion, and cause severe accidents that can jeopardize the continuous exploration and production. With the advance of new exploration and production technologies, oil and gas production has gone to deeper and over-pressured formations. These developments have enabled much improved growth in production, but also brought great challenges in scale management at high temperatures and pressures (i.e. above 200°C and 1,500 bar), which are commonly experienced in deep reservoirs. Under such harsh conditions, certain sulfide scales, i.e. ZnS, PbS, FeS, have been identified and become a particular issue associated with HPHT operations, in addition to common scales such as calcite, barite, and gypsum. These sulfide precipitates are often referred to as exotic scales [1] and are formed as a result of the dissolution of formation minerals and/or presence of hydrogen sulfide gas. They are especially prone to form when pressure and temperature change as brine, oil and gas are transported from underground to the surface, due to the decreased solubility as the condition changes. Damage resulting from increased occurrence of these exotic scales is exacerbated by high temperature, high pressure, and high brine concentration conditions. Understanding the chemistry involved in scale formation, gaining insights into the deposition of exotic scales, and reliably predicting the scaling tendency are therefore of vital importance to reduce the operational risk and to provide foundation for the development of effective scale remediation technologies in oil and gas recovery processes.
Complexity of “exotic” mineral scaling chemistry
The high complexity of the scale formation results from the large number of species present in the produced water, variations in brine composition, chemical reactions involving species present in the liquid, partitioning of volatile components between the liquid and vapor, and chemical equilibria between the solid phases and ions that are dissolved in solutions which govern the solubility of scale-forming solids. All of the above can be significantly affected by changing environmental conditions, i.e., temperature, pressure, and brine concentrations.
A characteristic behavior of the heavy metal ions, , , and , is that they undergo consecutive hydrolysis reactions to yield a series of hydrolyzed species (e.g. , , ) which strongly affect the pH dependence of the solubility. These sulfide deposits have been classified as pH sensitive scales [2], so that any issues that control the brine pH also affect their scaling tendencies. In the presence of complexing ions such as chloride ion, a common ion in the produced brine, these metal ions form chloride complexes (e.g. , , , and ) which can greatly impact the solubility and scaling tendency of the metal sulfides. In addition, they also form sulfide complexes (e.g. and ) which influence the solubility in the presence of and other sulfide species. Aside from the metal-containing species, the sulfide ions participate in aqueous reactions for the pH-dependent equilibrium transition between the , , and species. Furthermore, self-dissociation of water takes place in all aqueous environments, i.e., ; and volatile neutral species (e.g. and ) may partition between vapor and liquid phases or transition into liquid-liquid equilibria at elevated pressures. The chemical speciation and phase equilibria are strongly interconnected as temperature, pressure, pH, and solution composition change. The complexity of the aqueous media as well as the varying pressure and temperature conditions have made it a great challenge to accurately predict scale formation at extreme conditions. Such prediction is utmost important for the effective scale management in oil and gas flow assurance.
OLI simulation tools for sulfide scaling prediction
OLI Systems has developed a comprehensive thermodynamic model to predict scaling under conditions ranging from ambient to extreme. The model is based on OLI’s proprietary Mixed Solvent Electrolyte (MSE) thermodynamic framework and relies on a detailed treatment of speciation in the liquid phase [3]. The model has been designed to represent the solubility of scaling minerals at temperatures up to 300 °C and pressures up to at least 1,700 atm. The model considers the effect of multiple ionic solutes and dissolved gases on the solubility in systems that may form various competing solid phases. The model has been applied to sulfide scales including ZnS, PbS, and FeS in addition to the most common oilfield mineral scales (e.g. calcite, barite, celestite, anhydrite, gypsum, and halite). The OLI simulation Platform V10 incorporates the model with a thermodynamic database containing all chemical species that are necessary to enable reliable scaling predictions under oil and gas production environments.
How reliable is OLI’s simulation for predicting “exotic” mineral scaling?
Established on a sound theoretical basis, the performance of the MSE thermodynamic model has been verified by analyzing an extensive amount of experimental data and solubility trends in terms of temperature, pressure, and environmental composition. It accurately reproduces the solubility of a variety of mineral scales in water and in multicomponent brines ranging from dilute to highly saline (typically up to ~6 m Cl) and the effects of temperature, pressure, and nonaqueous solvents on mineral scaling [4,5]. Specifically, the model reproduces scale formation of sulfide minerals including PbS, ZnS, and FeS, as well as most common oilfield mineral scales over wide ranges of conditions. Figure 1 demonstrates the effect of partial pressure of sour gas , , on the solubility of PbS in a wide range of temperature. Increased leads to an increase in solubility, which is a direct consequence of the solution chemistry and is a manifestation of the formation of aqueous Pb-S complexes. The effect of the total pressure on the PbS solubility is shown in Fig. 2 at a fixed environment composition (e.g. 4 molal of NaCl) and the solubility is seen to have a moderate increase with pressure. Results in Figures 1 and 2 show increased solubility with temperature where the model reproduces the data within experimental scattering.
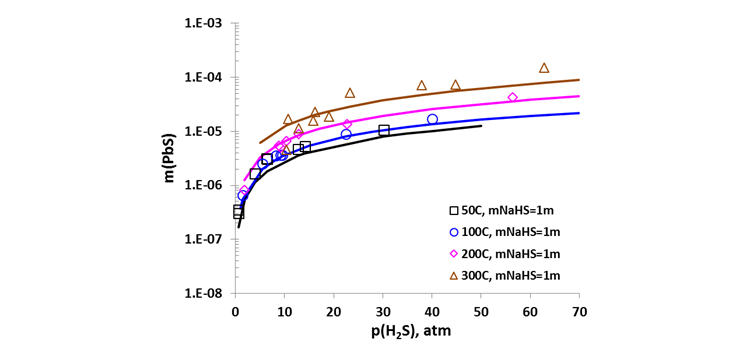
Figure 1. Calculated (curves) and experimental (symbols) solubility of PbS: Effect of partial pressure and temperature in a 1 m NaHS solution.
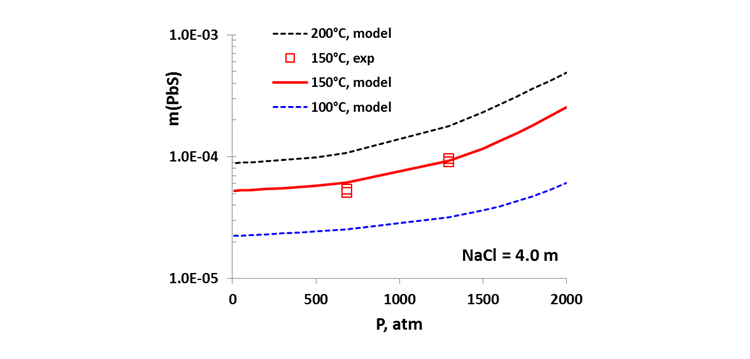
Figure 2. Calculated and experimental solubility of PbS: Effect of total pressure in a 4 m NaCl solution
Figures 3 shows a comparison of predicted and experimental pH dependence of ZnS solubility over a wide pH range at 150°C. The results are both at vapor-liquid saturation (Psat) and at high pressures up to 1305 atm. The model reproduces experimental results within the uncertainty of these data.
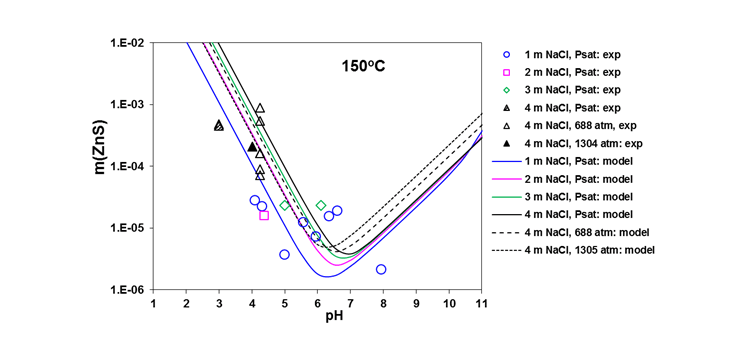
Figure 3. Solubility of ZnS at 150 °C in NaCl solutions along vapor-liquid saturation and at high pressures.
Demonstrated in Fig. 4 are the solubility of mackinawite (FeS) as a function of pH and . It also shows the effect of complexation on the solubility. The MSE model takes into account the Fe-S complexes (e.g. and ) in addition to other Fe species (e.g. Fe+2 and hydrolyzed species) which may coexist depending upon the temperature and pH. With these complexes, the solubility has been accurately reproduced in the complete pH range. Without the complexes, the calculated solubility is reasonable only in acidic solutions (dashed lines).
Benefits of OLI’s simulation of mineral scaling for oil and gas industry
The MSE model enables simulating chemical speciation and predicting mineral scaling simultaneously in complex systems to provide insights for a greater level of understanding of the underlying chemistry that may affect the scale formation. The information obtained from the OLI simulation is of great value in the development of effective mitigation strategies of oilfield mineral scaling so that improved well productivity and reduced costs of scaling control can be achieved for maintaining the economic viability of the operations.
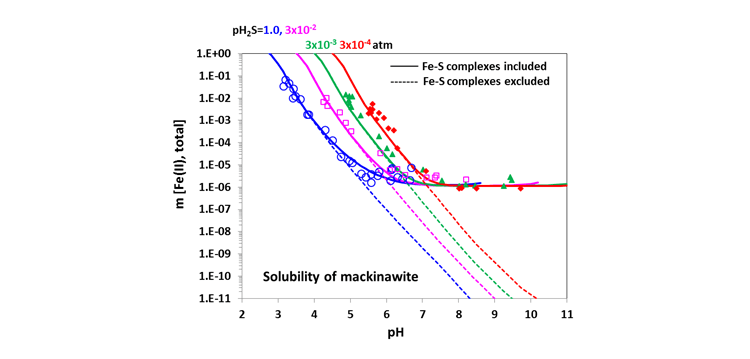
Figure 4. Solubility of mackinawite (FeS) at 23°C as a function of pH and calculated by including (solid lines) and excluding (dashed lines) Fe complexation, respectively, in comparison with experimental data.
What tools are available for predicting mineral scaling?
The OLI System’s thermodynamic property package, which implements the MSE model with the capability of scale prediction is available in OLI Studio V10 and OLI Flowsheet ESP V10.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- Savin, A. J., Adamson, B., Wylde, J. J., Kerr, J. R., Kayser, C. W., Trallenkamp, T., Fischer, D., Okocha, C.: Sulfide Scale Control: A high efficacy breakthrough using an innovative class of polymeric inhibitors. SPE International Oilfield Scale Conference and Exhibition, Scotland (2014).
- Olajire, A. A.: A review of oilfield scale management technology for oil and gas production, Journal of Petroleum Science and Engineering 135 (2015) 723–737.
- Wang, P., Anderko A., Young R. D.: A speciation-based model for mixed-solvent electrolyte systems, Fluid Phase Equilibria 203 (2002), 141-176.
- Anderko, A., Wang, P., Springer, R. D., Lencka, M. M., Kosinski, J. J. Prediction of mineral scaling in oil and gas production using a comprehensive thermodynamic mode, CORROSION 2010, paper no. 10129, San Antonio, TX, 2010.
- Lencka, M. M., Springer, R. D., Wang, P., Anderko, A.: Modeling Mineral Scaling in Oil and Gas Environments up to Ultra High Pressures and Temperatures, CORROSION 2018, paper no. 10828, 15-19 April, Phoenix, AZ, 2018

