OLI Systems has established critical materials processing as a central pillar in its mission to promote sustainability, making it one of the company’s top five focus areas. Recycling lithium-ion batteries (LIB) aids critical materials processing by recovering valuable materials and minimizing environmental impact. Recycling LIB is crucial for supply chain security, especially given their widespread use in electric vehicles, portable electronics, power tools, renewable energy storage, etc. If LIB fails, efforts should be made to repair it. Refurbished LIB can be repurposed for various uses. Recycling should be the final step in the process. While the anode of LIBs typically consists of graphite, graphene, Si, lithium titanate/lithium titanium oxide (LTO), or Cu-based materials, the cathodic waste materials of LIBs often contain valuable metals such as Li, Co, Ni, Mn, Mg, etc. These elements can potentially be extracted and separated from the liquor of spent LIB cathode scrap.
Solvent extraction (SX), also referred to as aqueous/organic liquid-liquid extraction, is a crucial step in the hydrometallurgical recovery of LIBs, enabling the recycling of critical metals such as Co, Ni, and Li at ambient temperatures. In hydrometallurgical extraction processes for metals like Co, Ni, and Li, several commercial extractants are commonly used. Notable examples include D2EHPA, Cyanex 272, and PC88A. These extractants are often combined with organic solvents—such as kerosene, n-heptane, iso-octane, or toluene—to enhance the solubility of the metal-extractant complexes in the organic phase. Developing a chemistry database for metal extraction pH isotherms is crucial for accurately predicting distribution ratios, determining the necessary extraction stages, and developing a complete SX process model.
OLI’s V12 introduces a new database for solvent extraction that includes various extractants and solvent chemistries (see Figure 1a), which can be coupled with a simulation software to optimize and scale up the hydrometallurgical recycling process of LIBs. The liquid–liquid extraction process and the metal-complex distribution in aqueous (A) and organic (O) phases could be affected by several factors such as pH, concentration of the extractant, temperature, A/O ratio, solvent-to-feed ratio, etc. Accurate modeling of metal partitioning between the aqueous and organic phases during the SX process requires precise estimation of speciation and activity coefficient parameters for the extractant, solvent, and metal complexes in both liquid phases. OLI’s Mixed-Solvent-Electrolyte (MSE)1–3 thermodynamic model, with carefully determined and validated parameters, allows for reliable predictions of the behavior of the metal-extractant complexes and all other species involved in the SX process. Figure 1b provides an example of how the predictions compare to the observed values for the percentage of metal extracted. This parity plot contains all data pertaining to Li, Co, or Ni using different extractants (D2EHPA, Cyanex 272, PC88A, TBP, etc. at different concentrations), in various diluents (kerosene, n-heptane, iso-octane, toluene, etc.), across temperatures ranging from 20 to 60 °C.
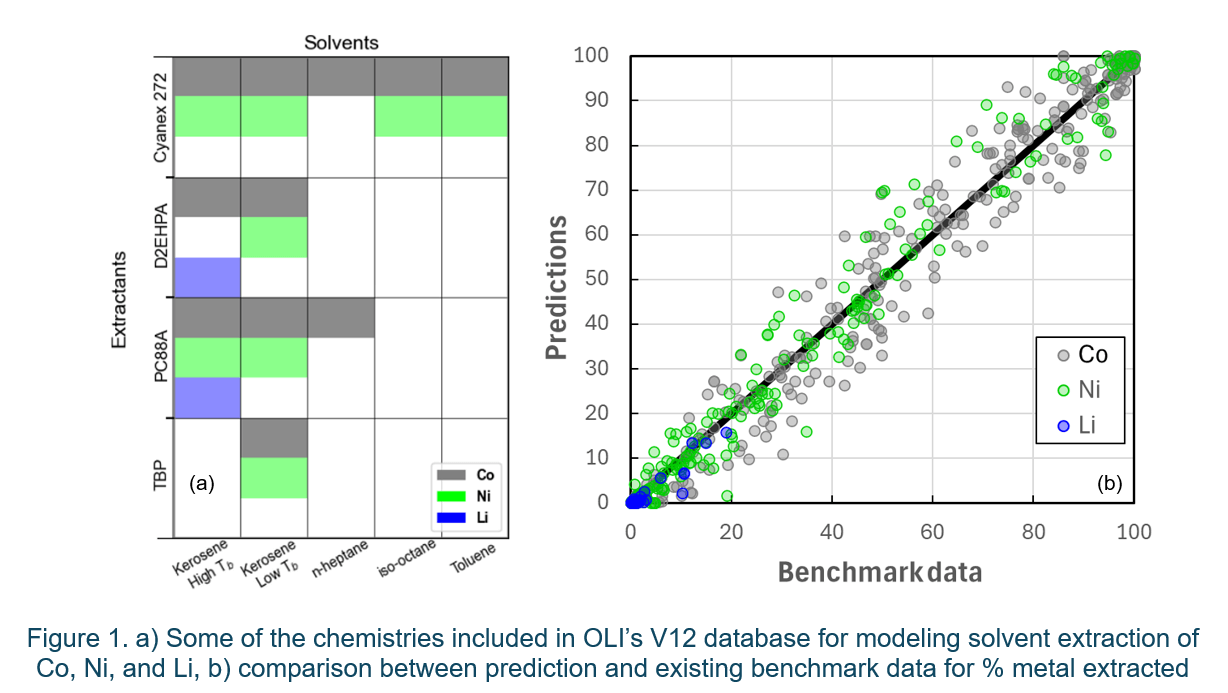
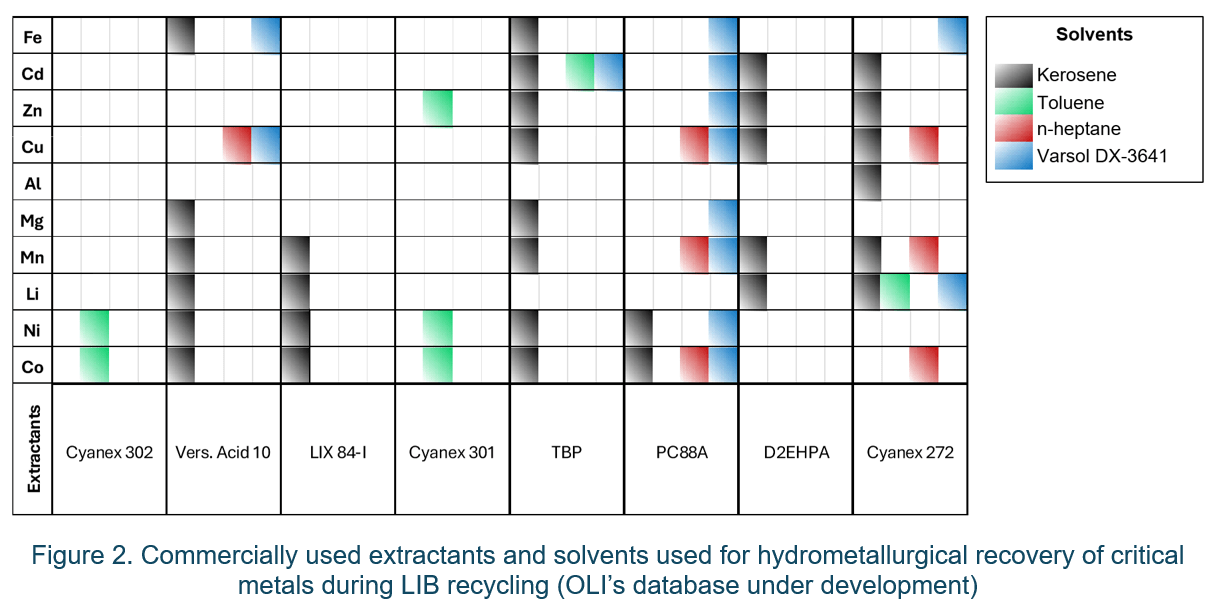
The OLI software V12 also includes other commonly used extractants, such as Cyanex 301 and Versatic Acid 10, and a database for incorporating chemistries applicable to the recovery of other high priority elements, such as Mn, Mg, Al, Cu, and Zn, is under development (Figure 2). This will provide the capability to optimize the operating conditions for a standard hydrometallurgical process for metal recovery from the black mass of shredded spent LIBs.
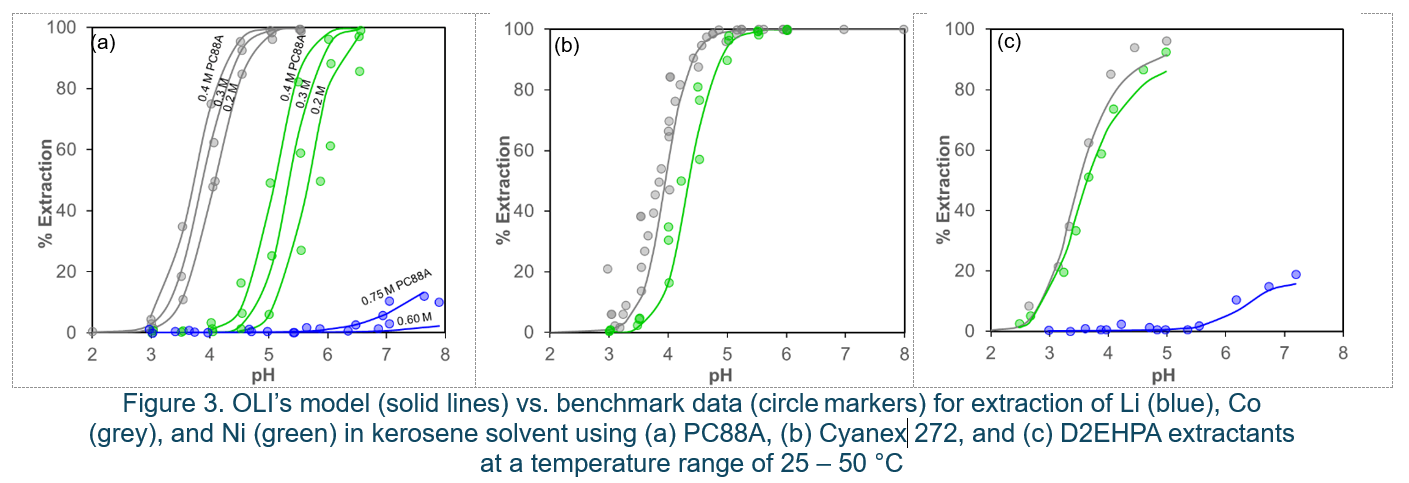
Equilibrium pH largely influences the extraction of metal ions, as it changes the solubility of the polar solvent, ionization state of the functional groups on the extractant molecules, and ultimately affects the speciation. Therefore, most efforts have focused on determining the influence of operational pH on the recovery and separation of metals from a sample composed of a mixture of waste household batteries. Figure 3 illustrates several examples of predicted extraction isotherms for Co, Ni, and Li using the extractants PC88A, Cyanex 272, and D2EHPA in kerosene. The model predictions show a reasonable level of agreement with the experimental isotherms. The extraction of Co and Ni significantly increases with rising pH, particularly between pH 4.5 and 5. Li does not extract at pH levels below 5.5; Li extraction into the organic phase starts at pH levels above 5.5 to 6. This is because, at higher pH levels, the formation of lithium hydroxide and Li-extractant monomers in the organic liquid is thermodynamically more favorable. This shift in favorability leads to a greater presence of these compounds in the organic phase, enhancing the extraction efficiency. The MSE speciation-based model in both O and A liquid phases accurately captures these complex process behaviors during the SX of Co, Ni, and Li.
Looking ahead, we aim to expand the SX database to include additional high-priority critical metals (as shown in Figure 2) and to create a generalized model capable of simulating SX in complex multicomponent systems, where the feed is typically contaminated with high concentrations of various electrolyte residues. Additionally, our database expansion aims to incorporate synergistic solvent extraction (SSX), which uses two or more extractants in a single batch to recover multiple metals simultaneously. This approach enhances the selectivity and stability of the extracted metal complexes in the second liquid, ultimately improving extraction efficiency. Table 1 outlines OLI’s initiatives for database expansion and their benefits to sustainability, highlighting our commitment to advancing sustainable practices in critical material processing area as one of our central missions.
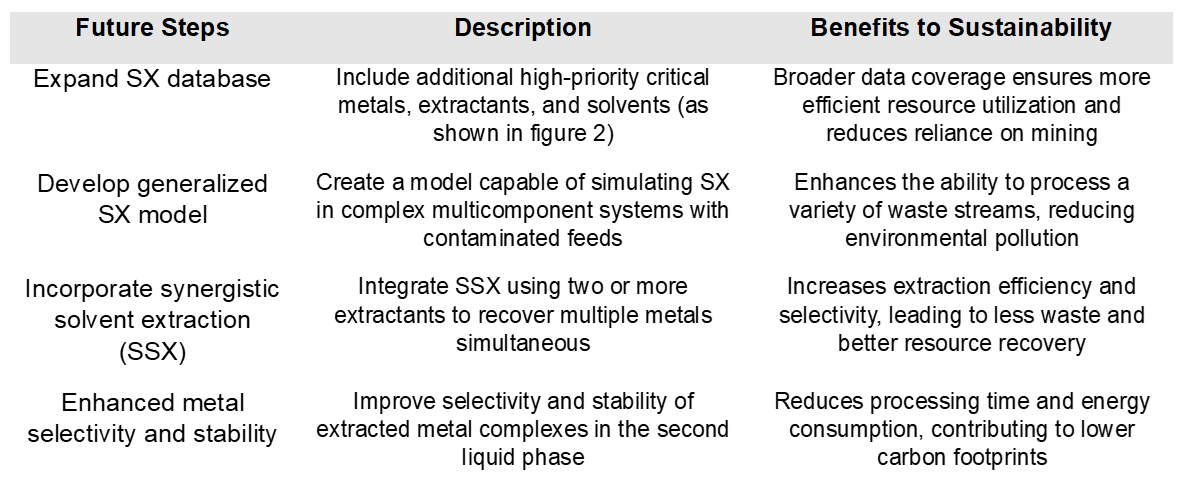
Table 1. OLI’s future sustainability efforts related to SX process and objectives for database development
For more insights on this topic, be sure to check out a related blog post (read here) by a fellow expert. Learn how OLI’s solutions can improve process simulation models for recovery of critical metals—contact us
References
- Wang, A. Anderko, R.D. Young, A speciation-based model for mixed-solvent electrolyte systems. Fluid Phase Equilibria 2002. 203: p. 141-176.
- Wang, A. Anderko, R.D. Springer, R.D. Young, Modeling phase equilibria and speciation in mixed-solvent electrolyte systems: II. Liquid–liquid equilibria and properties of associating electrolyte solutions. Journal of Molecular Liquids 2006. 125: p. 37-44
- Wang, R.D. Springer, A. Anderko, R.D. Young, Modeling phase equilibria and speciation in mixed-solvent electrolyte systems. Fluid Phase Equilibria, 2004. 222: p. 11-17.

