The growing demand for electric vehicles and renewable energy storage has intensified the need for efficient and sustainable recycling of lithium-ion batteries (LIBs). OLI Systems addresses this challenge by introducing a novel solvent extraction (SX) database integrated with our robust MSE thermodynamic framework. This blog post explores the capabilities of this database in optimizing hydrometallurgical processes for LIB recycling.
The widespread adoption of electric vehicles (EVs) and portable electronics has created a pressing concern: the long-term availability of critical battery materials. While lithium-ion batteries (LIBs) power these advancements, their spent forms pose a growing environmental challenge. To ensure a sustainable supply chain, efficient and environmentally friendly battery recycling processes are essential.
Solvent Extraction: A Cornerstone of LIB Recycling
Solvent extraction (SX) is a well-established and cost-effective method for recovering valuable metals like cobalt and lithium from spent LIBs. However, a significant hurdle in optimizing and scaling-up these processes has been the lack of a reliable thermodynamic model for process simulation tools. This often necessitates time-consuming and expensive experimental trials.
OLI Systems has established a long history of success in modeling applications like lithium extraction and purification from complex brines. Our comprehensive thermodynamic database provides a solid foundation for accurately predicting complex chemical behaviors, such as solvent extraction, offering the industry a more complete modeling solution.
A Novel Approach to Solvent Extraction Modeling
The newly developed solvent extraction database leverages our MSE (mixed solvent electrolyte) model, known for its ability to predict the chemical equilibria present in complex liquid-liquid systems. This database focuses on the recovery of critical battery metals like lithium, cobalt, and nickel. It incorporates essential components for SX modeling, including:
- Commercially relevant extractants (D2EHPA, Cyanex 272, PC88A)
- Commonly used solvents (kerosene, n-heptane, iso-octane, toluene)
- Phase modifiers (TBP)
The database development involved a deep analysis of the underlying SX chemistry, considering:
- Formation and stability of metal complexes
- Thermophysical properties of these complexes
- The influence of operating conditions (pH, temperature) on these factors
Validation and Model Performance
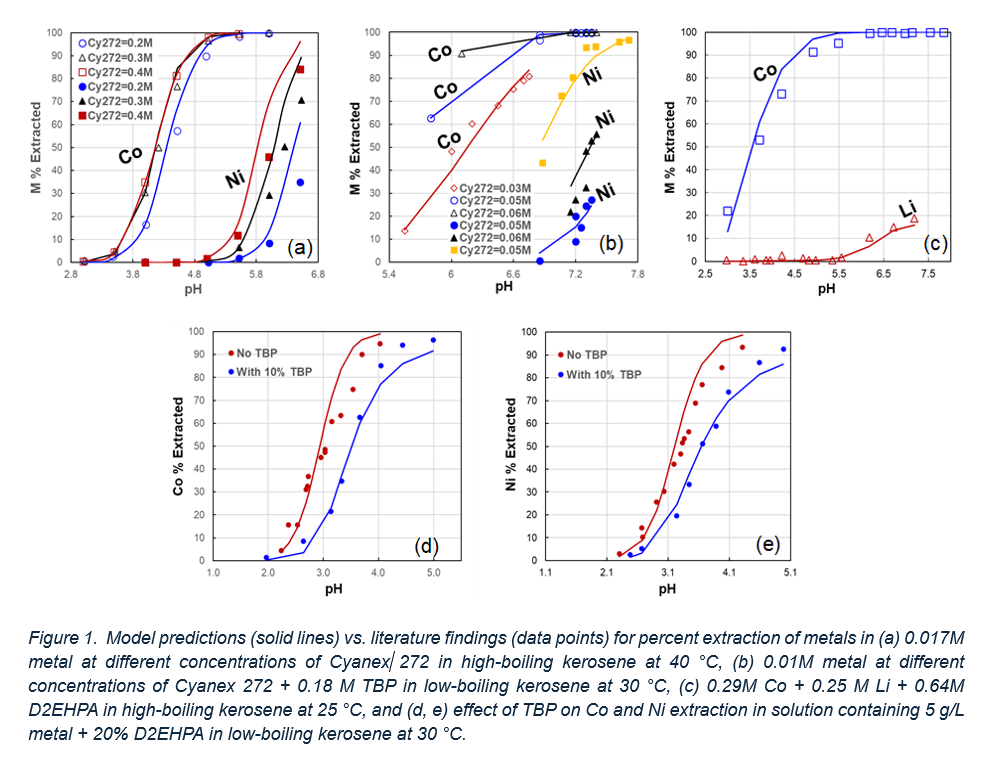
A critical aspect of SX modeling is the ability to reproduce extraction pH isotherms for predicting distribution ratios, extraction stages, and the overall SX process flowsheet. Figure 1 shows a sample of these isotherms for Li, Ni, and Co using DHEPA and Cyanex 272, with and without TBP. The data points represent literature values and demonstrate a high degree of agreement with the model predictions (solid lines).
Optimizing Hydrometallurgical Recycling with OLI’s Database
The recovery of valuable metals from spent LIBs involves a multi-step process often encompassing dismantling/shredding, pyrometallurgical, and hydrometallurgical stages. The MSE framework excels at modeling hydrometallurgical processes due to its applicability in electrolyte thermodynamics across various solvents. However, optimizing these processes to maximize metal recovery and minimize environmental impact, particularly those involving solvent extraction chemistry, has remained a challenge.
Our novel database accurately models these chemical interactions that occur during solvent extraction. This capability allows for predicting the efficiency of separating specific metals from the complex mixtures found in spent battery leach solutions. Additionally, the database enables the simulation of various operating conditions, facilitating “what-if” analyses to optimize solvent selection and process conditions for reduced solvent consumption and waste generation.
Competitive Advantage: The Power of the MSE Model
Unlike other modeling tools, our database is built upon a robust thermodynamic framework, the MSE model. This model predicts the complex chemical equilibria present in multiphase systems, a crucial capability for accurate battery recycling process modeling. This translates to more reliable predictions and ultimately, a more efficient and sustainable battery recycling process.
Looking Ahead: Continuous Improvement and Expanding the Database
By seamlessly integrating with the MSE framework, this database enables accurate modeling of complex chemical interactions during solvent extraction. This translates to significant benefits for designing and operating recycling processes, including:
- Predicting extraction efficiency for critical battery materials
- Optimizing process conditions for maximum metal recovery and minimal waste
- Minimizing the environmental footprint of the recycling process
We are committed to continuous improvement. The SX database will be expanded to encompass the recovery of additional high-priority materials like rare earth elements, manganese, and copper. Our goal is to develop a comprehensive model that can handle the complexities of multicomponent systems encountered in real-world battery recycling. This will involve incorporating a wider range of solvents and diluents as data becomes available. By continuously refining our database, OLI Systems remains dedicated to supporting the development of efficient, sustainable, and environmentally friendly battery recycling processes. For more information contact us.

