With the release of major versions (like V12) or revisions (like 11.5), OLI continues to make significant additions to the OLI platform’s chemistry database. Updates bring important changes, and this release is no exception. When we released V11.5 in November 2022, the database contained 134 new species. V12 surpasses this, introducing 213 new species.
These species fall into a few categories, expansion of the existing periodic table capabilities, like for iron, selenium, and phosphate or in strengthening density or transport predictions of existing species. Then there are expansions based on the need to cover new areas, like rare earth elements, battery materials (Li, Ni, Co, Mn), Solvent extraction, and Scale Inhibition. in areas that if of interest, have links that you can follow. Lastly, there is breakthrough work, MSE Corrosion. Such work required new theories as well as detailed data regression to create.
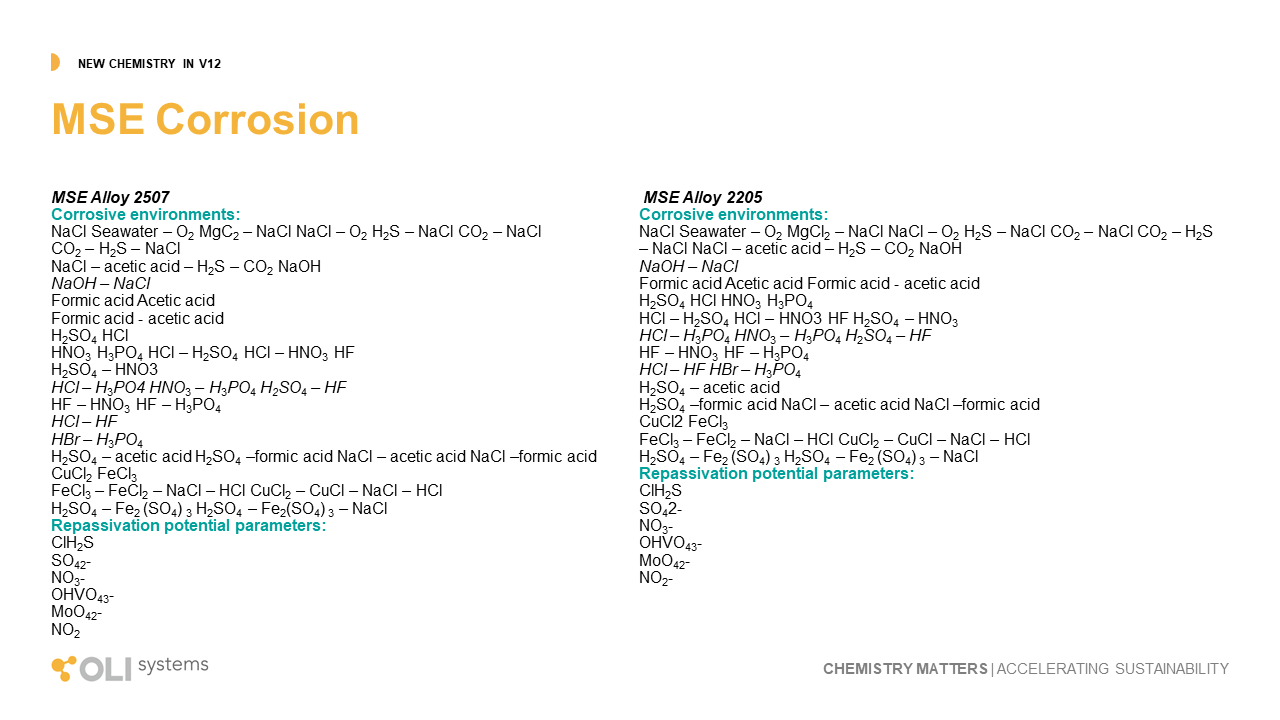
MSE Corrosion
Perhaps the most transformative work completed in the last two years is developing and validating a new corrosion mode, MSE corrosion. MSE corrosion is in the simplest terms, a tool that predicts corrosion under all process compositions. All models to date, including OLI’s current AQ corrosion model, requires water to be present in large relative quantities to compute. That leaves out an enormous number of corrosion environments, like concentrated H2SO4, ethanol, and supercritical CO2. MSE corrosion now makes it possible to understand the corrosivity of these environments under process conditions.
This is the first release of MSE corrosion, and during subsequent releases, we shall see further advancements in materials and chemistry. In this release, we include Alloy 2507 and 2205 in thirty-eight separate environments. This includes the effects of oxygen on their pitting potential.
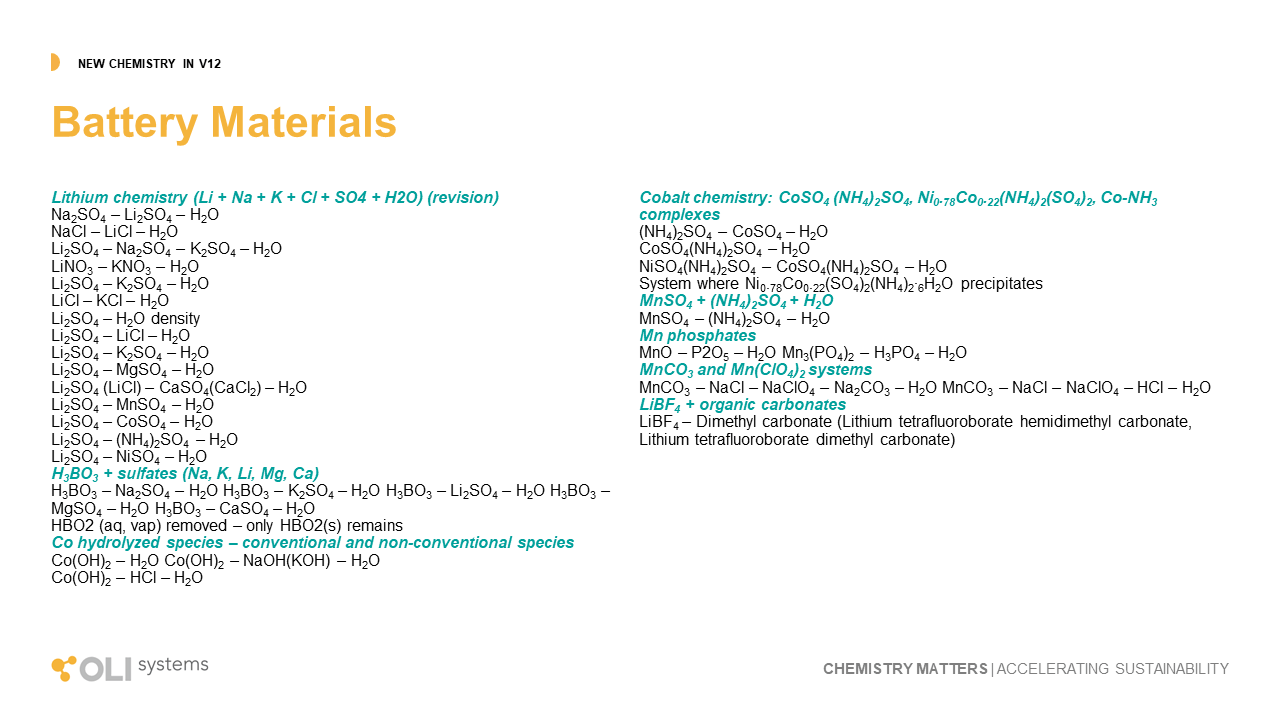
Battery Materials
OLI is being pulled into a very important industry, battery production. Our ability to predict the inorganic and organic chemistry of these materials make us critical to the industry’s development. A key area of research was on the lithium sulfate and borate systems. The sulfate chemistry is critical in acid extraction processes, while the borates are found in large concentrations within salars and geothermal brines. Also, since lithium is a minor metal in these natural brines, the work included the chemistry of K, Na, and Mg with these anions.
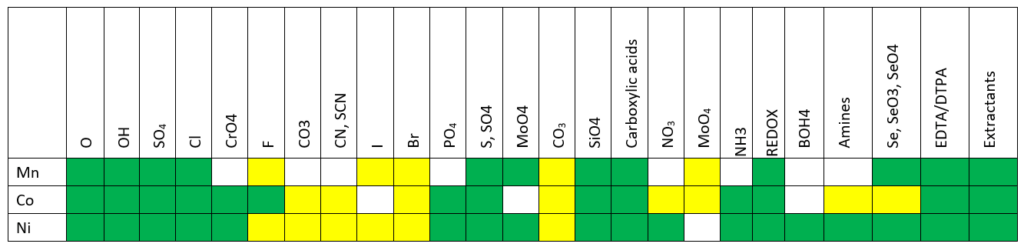
A second key area of battery materials is Cobalt, Nickel, and Manganese. V12 contains new and upgraded chemistry for these metals in contact with sulfate, ammonia, phosphate, carbonate, and hydroxide. Expansion of this chemistry allows for better predictions of battery recycling systems and the hydrometallurgy processing of new ores.
Lastly, our team began the work to include battery electrolyte chemistry. We completed the chemistry for LiBF4 with organic carbonates. Clients and researchers have a critical need to understand the properties of the electrolyte material and the V12 deliverable provides the start of that capability.
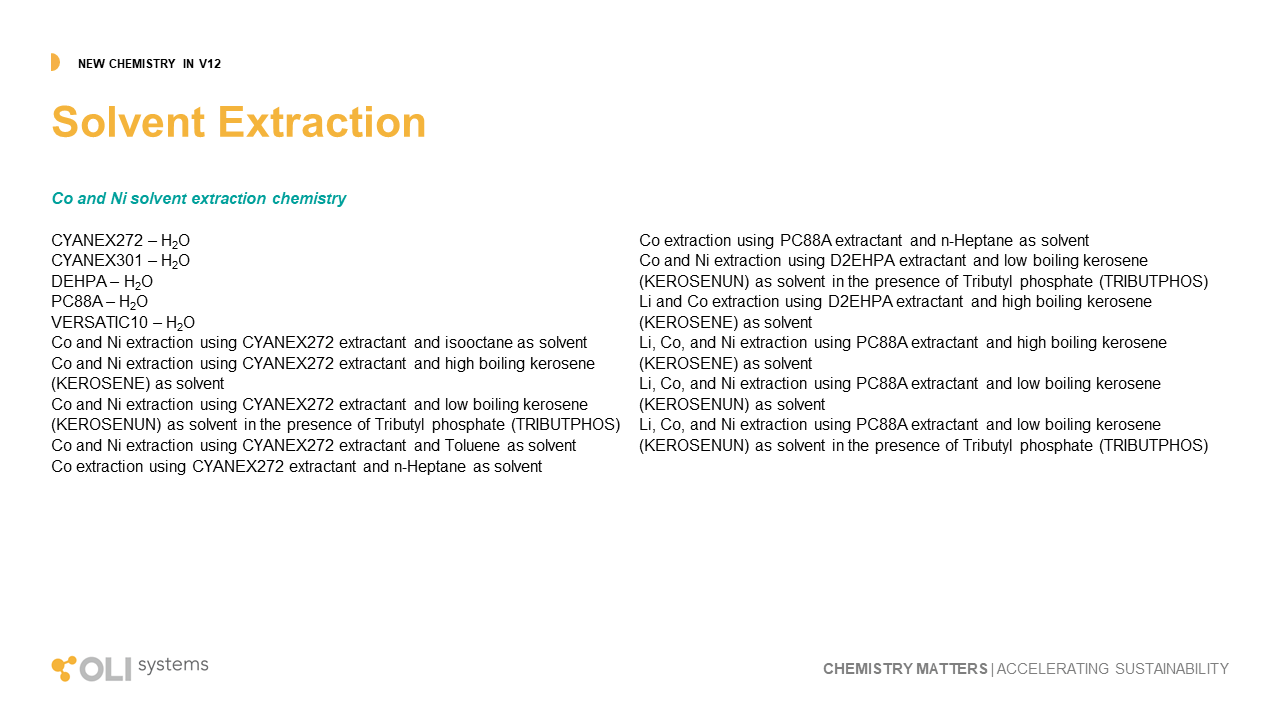
Solvent Extraction
Solvent extraction is a critical method for separating and purifying critical materials. Ni, Co, Rare Earth elements, are all examples of where this liquid-liquid extractive technology is useful, and in many cases the only pathway to produce purified materials. Starting with V12, OLI is bringing solvent extraction to the market. We have begun with five extractant chemicals, Cyanex272, Cyanex301, DEHPA, PC88A, and Versatic10. We include two kerosene diluents, low and high boiling point. And lastly, we have completed some of all of this work for Ni, Co, and Li. The impact of this research was presented at ALTA ( June 2024, Perth AU). We showed how OLI V12 chemistry and engineering can simulate the conversion of black mass to purified materials. A complete list of solvent extraction work in V12 is shown below.
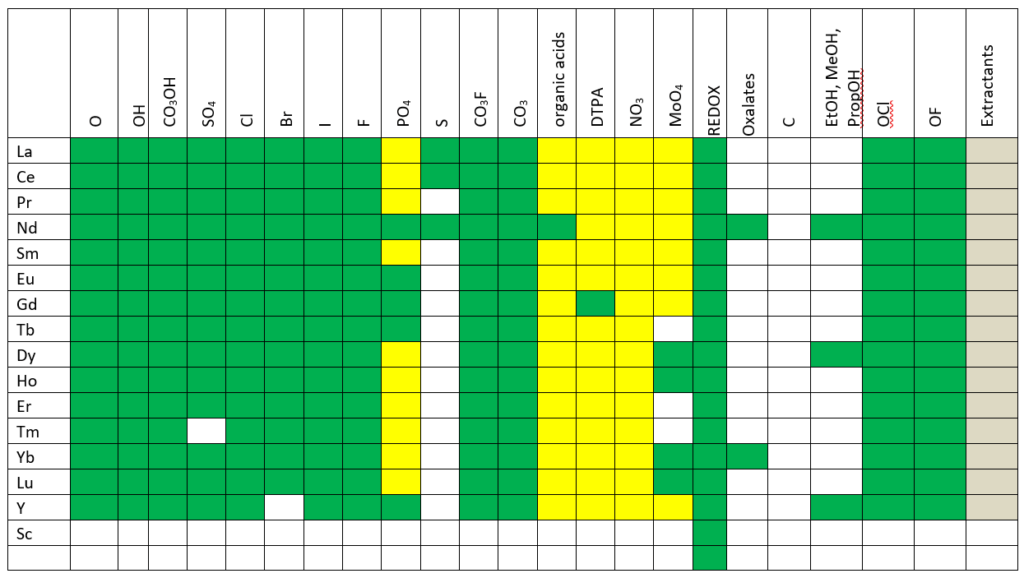
Rare Earth Elements
OLI continues to develop the world’s only comprehensive treatment of rare earth elements. This work started in 2011 when OLI was selected by the USDOE to work with the Critical Materials Institute and quantify through modeling, the research accomplished through that institution. We continue to take data from them and the existing literature to build out the Rare Earth systems. This version provides new chemistry for the fifteen REY (rare earth plus yttrium) with hydroxycarbonate, carbonates, flurocarbonates, oxychlorides, and improvements in existing hydroxides and phosphates.
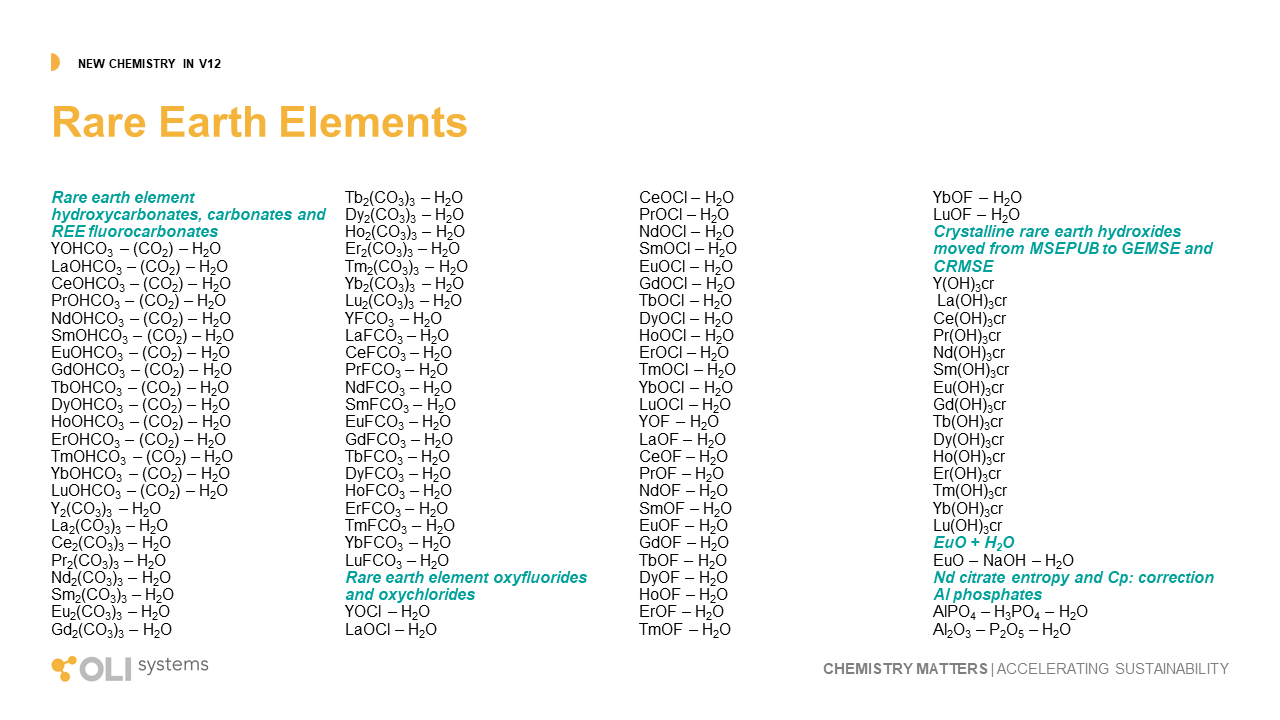
The image below shows where the database now is in modeling rare earth chemistry.
Key
Green – complete treatment of solution chemistry and solid phases in MSE.
Yellow – approximate treatment is available in the AQ model, work remains to be done in MSE.
Gray – estimates completed in private databases.
White – work still needs to be done.
Scaling Kinetics and Inhibition
Eighteen months ago, OLI released a comprehensive model that predicts induction times of key mineral scales. We have followed that up with a revision to the Classical Nucleation theory used in our original work. This theory revision now predicts a continuous curve between homogeneous and heterogeneous nucleation. It eliminates the ambiguity that exists between which mechanism is important to scaling within a process. The upgraded database is for the following minerals, CaCO3, BaSO4, CaSO4.2H2O, and SrSO4 and for the following Inhibitors HEDP, DTPMP, NTMP, PMLA, EDTMP, and PBTC.

Carbon Capture
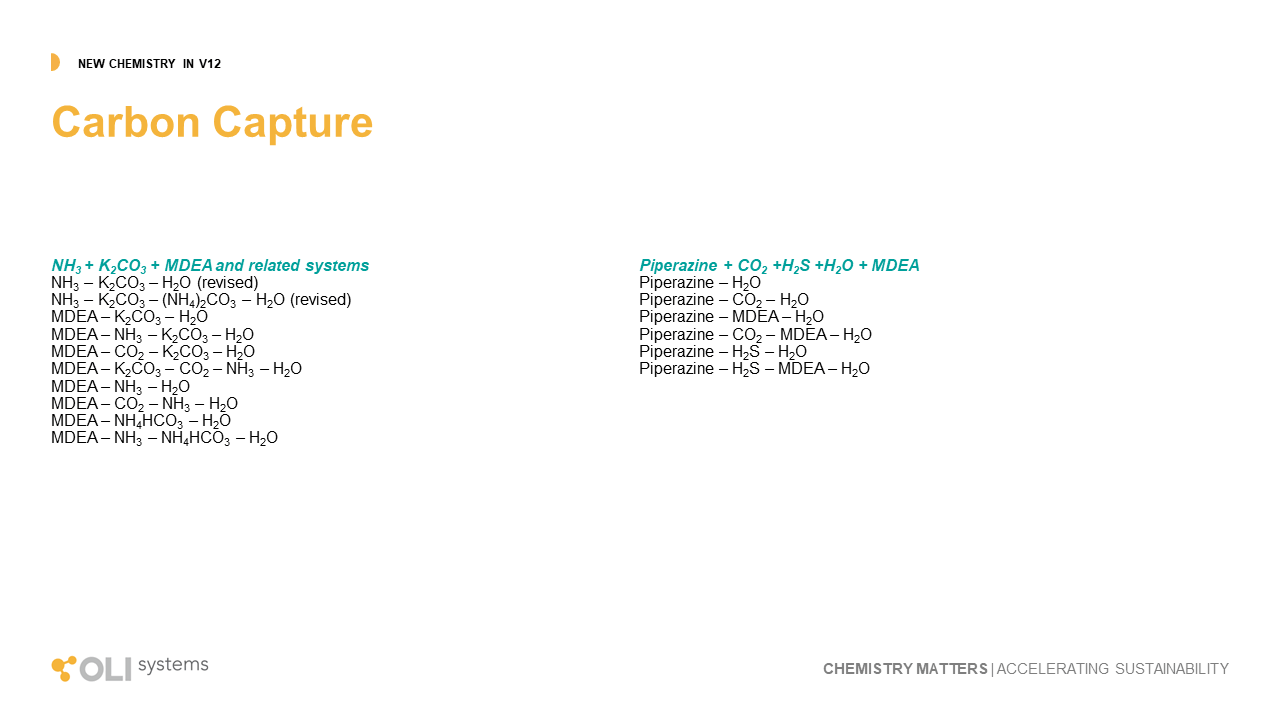
Much of OLI’s research is in the new sustainable economy as described with the Battery materials, Solvent extraction, and rare earth elements. It continues with our work in Carbon capture chemistry. There are many novel paths to remove CO2 from the atmosphere and from point sources, and all require a fundamental understanding of the chemical reaction in the absorption tower and the subsequent regeneration of the absorbing media. OLI continues to work in this area in two parallel paths. The first is via inorganic absorption, primarily the mixed-salt process using KOH-NH3, and the amine process, using a variety of neutralizing amines. We have expanded the chemistry for both paths with this release, for the amine process, we have added piperazine, and for the mixed-salt process we’ve incorporated MDEA. These are key improvements in both the applied research in this area, and also in the demand from client companies.
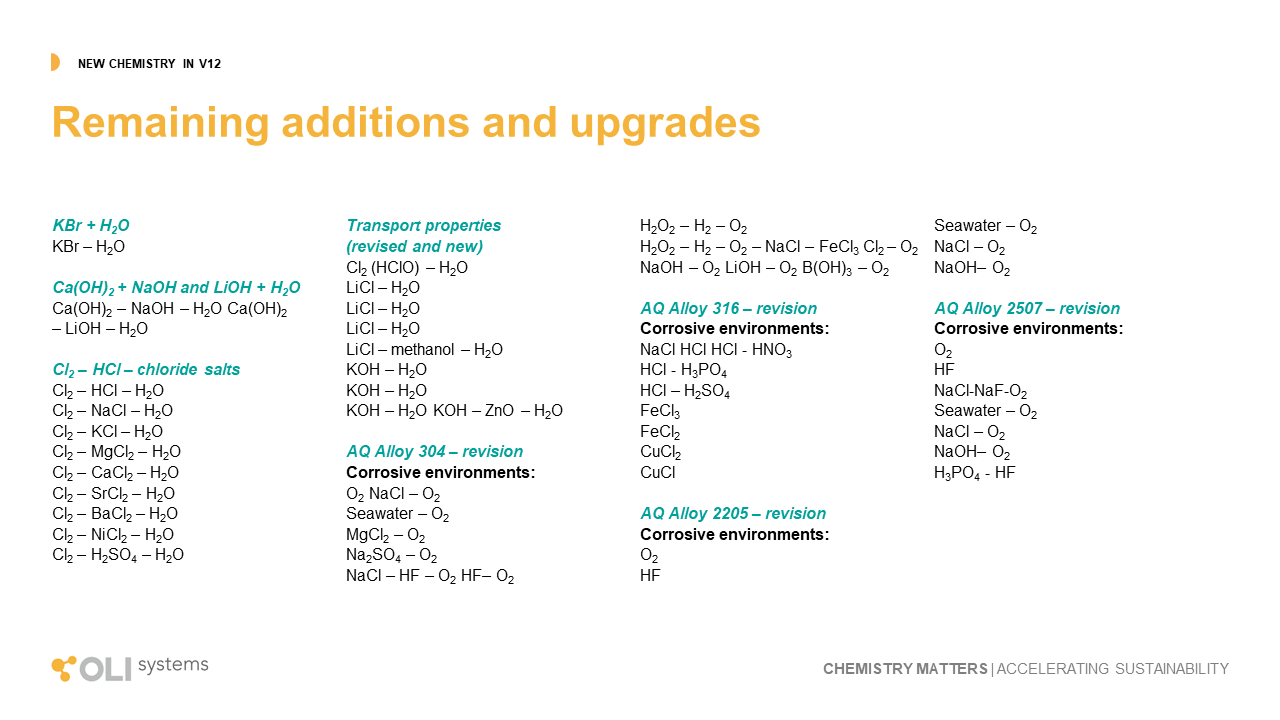
Other additions
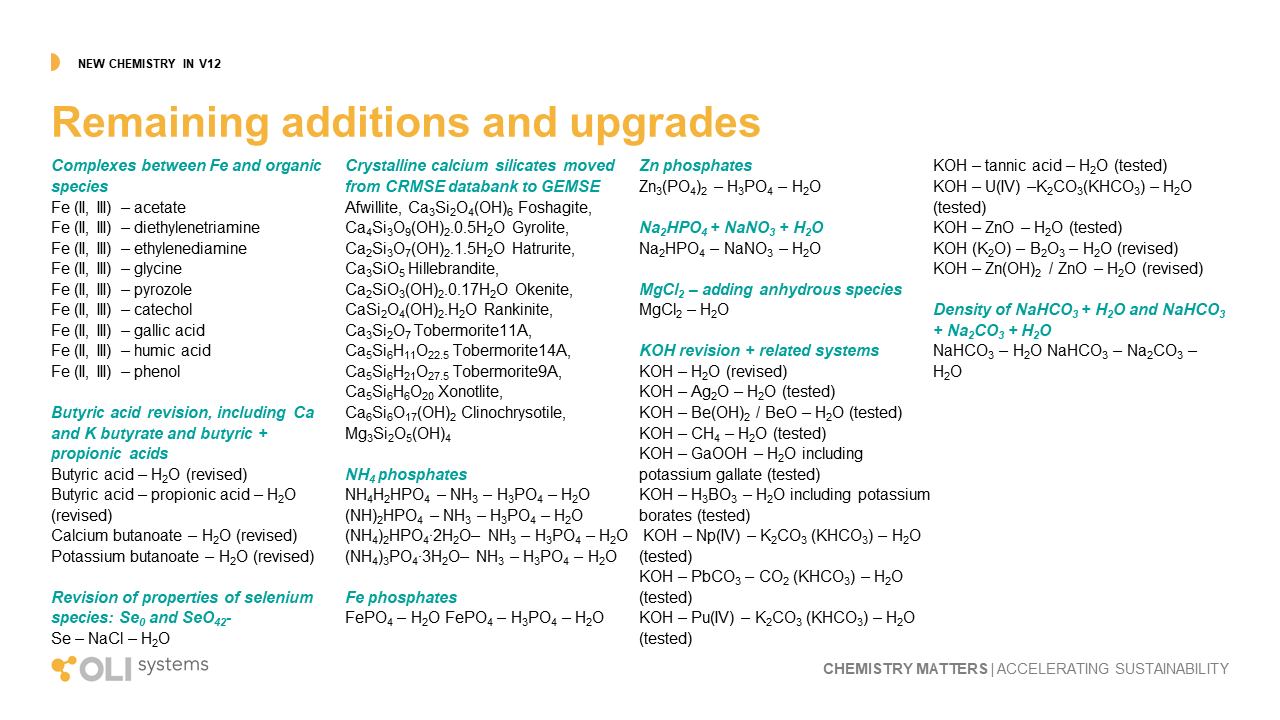
The remaining upgrades to the OLI database can be viewed using this link. It is a combination of improvement to existing chemistry and new chemistry entirely. The improvements cover changes to predicted values for properties like solubility, density, viscosity, vapor pressure, corrosion rates and other key outputs. The new chemistry expand our capabilities in borates, organic acids, metal-silicates, and metal-organic complexes.
To learn more about the OLI database please contact us.

