Harnessing the power of mixed-solvent electrolyte (MSE) thermodynamic model in corrosion prediction
Corrosion poses significant challenges to structural integrity and material longevity across various sectors, including infrastructure, transportation, and manufacturing. It can lead to both significant economic losses, estimated to cost about 3-4% of global GDP, and pose serious safety risks. Effective management and mitigation of corrosion are essential, necessitating advanced predictive tools and modeling techniques to foresee corrosion-related failures and enhance asset integrity.
Complexity of corrosion modeling
Corrosion modeling presents significant challenges due to the complex interplay of numerous factors that influence corrosion. These include the metallurgy of the material, the chemistry of the solution, and environmental variables such as temperature, pressure, and flow conditions. Accurately predicting corrosion behavior is complicated by the fact that these variables can change dramatically across different environments and conditions, making experimental data insufficient to cover all potential scenarios. Simple extrapolation from limited data is often unreliable, thereby requiring more sophisticated modeling capabilities.
To accurately predict how metals interact with their environments requires detailed knowledge of thermodynamics of solutions, kinetics, transport phenomena, and adsorption characteristics. Thermodynamics provides insights into the stability of different species in the system, helping to determine which electrochemically active species might be present under equilibrium conditions. Kinetics determines the rate at which corrosion processes occur, which cannot be accurately predicted by thermodynamic stability alone. Transport properties need to be known to predict the movement of ions and molecules to and from the metal surface, which can influence the concentration of corrosive agents. Lastly, how species adsorb on the metal surface affect both the kinetic rates of electrochemical reactions and the protective properties of surface films that may form. Collectively, these factors constitute a multidimensional challenge that requires sophisticated modeling approaches to understand and predict corrosion behavior accurately.
Introducing MSE Corrosion
The MSE corrosion model integrates the mixed-solvent electrolyte (MSE) thermodynamic model with a previously developed electrochemical module. In our previous model, the speciation and transport properties was predicted using an aqueous thermodynamic module, which was limited in its range of applicability to water-dominated systems. The MSE thermodynamic model is a much more general and comprehensive model, increasing both the accuracy and applicability range of corrosion predictions.
Mixed-solvent electrolyte (MSE) thermodynamic model
The MSE model is a comprehensive thermodynamic framework that accounts for the complex interactions between species in the solution. It employs a combination of long-range electrostatic interactions, short-range intermolecular interactions, and ionic interactions to calculate the excess Gibbs energy of the system. This approach allows the model to accurately predict the activities of species across a wide range of concentrations, from infinite dilute solutions to pure salt limit.
One of the key strengths of the MSE model is its ability to handle systems with several components with complex chemistries. This is especially important for systems which might have small amounts of impurities that can radically change phase behavior and concentrations of corrosive species in each phase as in the case of CO2 transportation. The model has been extensively validated for a wide variety of systems and conditions (see here for examples of applications).
The electrochemical kinetic module
While the MSE model provides the necessary thermodynamic foundation, the electrochemical kinetic module is responsible for simulating the partial anodic and cathodic reactions enabling it to model active corrosion, active-passive transition, and passive dissolution. The electrochemical model employs the Butler-Volmer equation to describe the kinetics of the active anodic dissolution and cathodic reactions. This equation relates the current density to the surface overpotential, taking into account the activities of the species involved in the reaction. Active-passive transition is introduced by considering a current that leads to the formation of a passive layer in addition to the current associated with active dissolution. The effect of solution chemistry on passive dissolution and active-passive transition is modeled by considering surface reactions between the oxide film and solution species. Such reactions result in the formation of surface complexes, which may either enhance or inhibit dissolution. Finally, mass transfer effects are quantified using flow regime-dependent mass transfer coefficients.
Illustrative example: Corrosion behavior of a duplex alloy in sulfuric + nitric acid system
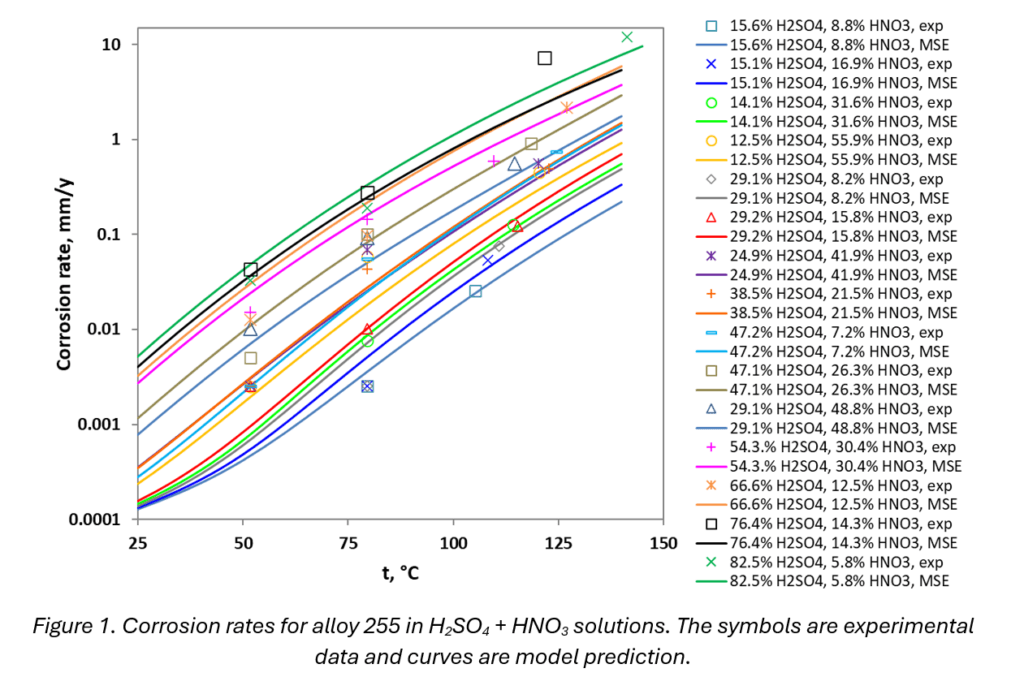
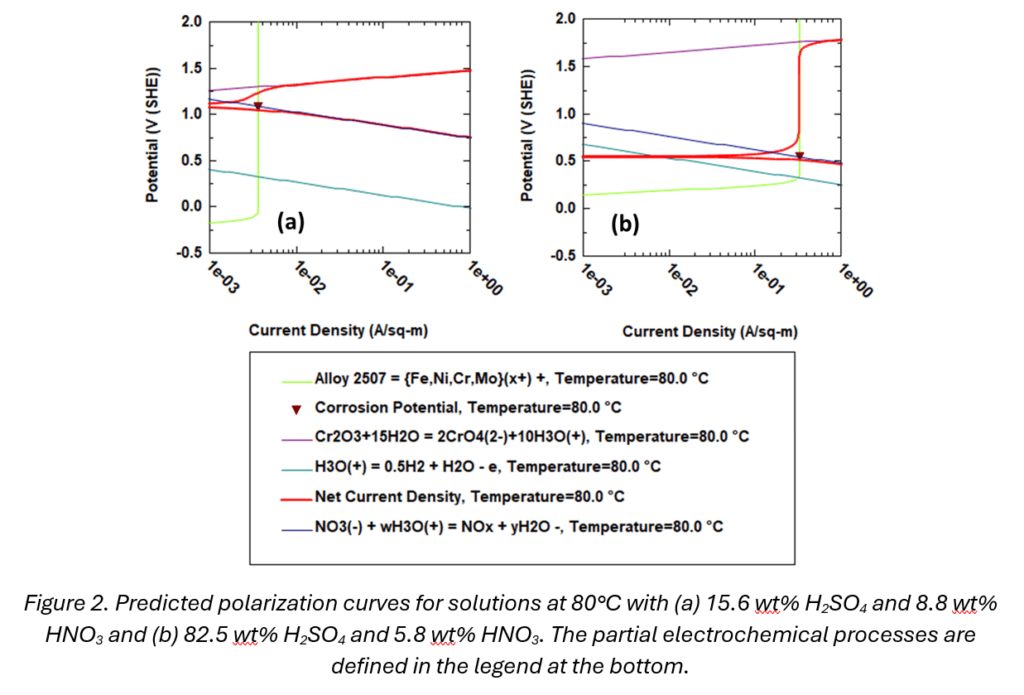
To demonstrate the practical application and benefits of the MSE corrosion model, consider the system combining sulfuric acid (H₂SO₄) and nitric acid (HNO₃). These kinds of mixtures are common in leaching processes during extraction and recycling. As shown in Figure 1, the model accurately reproduces both the temperature and concentration dependence of corrosion rates of alloy 255 in a wide range of conditions. The predicted polarization curves (Figure 2) for two specific systems – 15.6 wt% H2SO4 and 8.8 wt% HNO3 (shown as the cyan curve in Figure 1) and 82.5 wt% H2SO4 and 5.8 wt% HNO3 (cf. the green curve in Figure 1) at 80°C – reveal a wealth of information that cannot be obtained by just looking at the rate curves. The dominant cathodic reaction is the reduction of the nitrate ions (blue curve in figure 2) and the system is in the passive state as shown by the corrosion potential lying on the vertical part of the anodic curve. Both environments have similar concentrations of HNO3 but very different concentrations of H2SO4. The presence of H2SO4, which decreases the pH of the system, increases the dissolution rate of the passive oxide layer, and significantly increases the corrosion rate. The dissolution rate of the passive film strongly depends on the acidity of the system, which is quantitatively predicted by the MSE thermodynamic model. Thus, the corrosion rates can differ by almost two orders of magnitude at a given temperature depending on the acidity of the mixture (cf. Figure 1). The corrosion in highly concentrated acid solutions can be modeled only because of the applicability of the MSE model in this regime. This insight demonstrates the importance of considering the complex interplay of species in the corrosive environment.
Main takeaways
- Enhanced Accuracy in Speciation: MSE can quantitatively predict the speciation of ions in complex mixtures, crucial for understanding how different ions influence corrosion rates.
- Comprehensive Application Range: Unlike the old aqueous corrosion model, which had a limited applicability range, MSE can handle the entire concentration range, making it applicable across diverse industrial processes some of which were inaccessible to the older model.
- Mixed-solvent systems: Corrosion in mixed-solvent processes such as those involving methanol or glycol, common in industrial processes, can now be modeled within the MSE framework.
- Simulated Polarization Curves: MSE provides detailed polarization curves that provide crucial information about the state (active, passive, active-passive transition) of the system and the dominant cathodic reactions that could be helpful in designing mitigation strategies.
Conclusion
The MSE corrosion model represents a significant advancement in corrosion prediction and management. By accurately predicting speciation, handling a wide range of concentrations, and accounting for mixed-solvent systems, the MSE model enables a more comprehensive understanding of corrosion behavior in complex environments. This tool has the potential to transform corrosion management practices across various industries, leading to safer, more reliable infrastructure and significant economic benefits.
MSE corrosion model for two duplex stainless steels, alloys 2507 and 2205, will be available in OLI Corrosion Analyzer V12. For more information see here or contact us at https://www.olisystems.com/contact-us/.