Solvent extraction is an industry-accepted method for separating rare earth elements from pregnant liquor. We studied the potential for OLI Flowsheet: ESP to simulate the mass, chemistry and energy balance of an extraction process. OLI Systems, as a company, is not an expert in building or operating extraction plants and our acquired knowledge had to come from conversations with industry experts and reading literature.
We learned from this effort that the pregnant liquors contain many combinations of rare earth elements and impurities; the acids most used are nitrate and chloride; the common extractants are HEHEHP and D2EHP; and the diluent is an aliphatic kerosene; the extraction temperatures are somewhat ambient (25-50°C); the heats of reaction (elements partitioning to the diluent phase) is limited; and the key heat of reaction comes from the extraction “saponification” step (acid neutralization with caustic). We learned that extractants are used in combination to enhance separation, and that there is a competitive are of research to develop more efficient extractant chemistries.
Given the nature and complexity of this area, we began our work by studying systems where we could find the most information: separation of rare earth from a nitrate solution using HEHEHP at 25°C and using a n-C10 to n-C16 alkane mixture (primary components of kerosene). We did this “reconnaissance” work with two questions in mind; can the existing OLI Flowsheet:ESP tool simulate such a process, and if so, does the market have a need to model this step rigorously when using a process simulator to design and optimize their operations?
We often do such work before asking the OLI Thermophysical Modelling (TPM) and Software Development teams to get involved, in this way, we can determine if such efforts are a good investment for the company and our clients. Developing a comprehensive database or new engineering blocks is a huge undertaking, and so the Application Engineering team does surveillance work. We create a private database of predictions that are qualitatively consistent with the literature data and presenting it to the market. These private databases are generally created for a single temperature and for a select set of chemicals. We use existing blocks in OLI Flowsheet: ESP to simulate the process. If the work is successful; we can model the process effectively and the market needs these tools, then we request that the TPM team develop a comprehensive database for this chemistry and request that the Software Development team develop the proper engineering blocks needed to model it effectively.
Developing a qualitative database
The TPM team is currently developing an extractant database for the nickel-cobalt-manganese separation. We used techniques they had already developed for these and other chemicals to create a private database for HEHEHP (2-ethylhexyl phosphonic acid mono-(2-ethylhexyl) ester) and the fifteen rare earth elements and yttrium (REY). There are many papers that contains properties and extraction information for the HEHEHP REY-NO3 system. a great deal of this data is compiled by Qi in the book Hydrometallurgy of Rare Earth metals.
We settled on the six chemical reactions for the HEHEHP extractant.
HEHEHP0=EHEHP−1HEHEHP0=EHEHP−1
2HEHEHP0=(H0.5EHEHP)−12+H+2HEHEHP0=(H0.5EHEHP)2−1+H+
2HEHEHP0=(HEHEHP)022HEHEHP0=(HEHEHP)20
HEHEHP0(a)↔ HEHEHP0(o)HEHEHP(a)0↔ HEHEHP(o)0
(HEHEHP)02, (a)=(HEHEHP)02(o)(HEHEHP)2, (a)0=(HEHEHP)2(o)0
NaEHEHP0(o)+H+1(a)=HEHEHP0(o)+Na+1(a)NaEHEHP(o)0+H(a)+1=HEHEHP(o)0+Na(a)+1
…and five reactions for REY, HEHEHP (HA for brevity), and NO3.
RE+3+4HA0=RE(A)−1(a)+4H+RE+3+4HA0=RE(A)(a)−1+4H+
RE+3+3A−1=RE(A)03 (o)RE+3+3A−1=RE(A)3 (o)0
RE+3+6HA0=RE[(H0.5A)2]03,(o)RE+3+6HA0=RE[(H0.5A)2]3,(o)0
RE+3+3NO3−1+2HA0= RE(NO3)3(HA)02 (o)RE+3+3NO3−1+2HA0= RE(NO3)3(HA)2 (o)0
RE+3+NO3−1+4HA0= RE(NO3)[(H0.5A)2]0(o)RE+3+NO3−1+4HA0= RE(NO3)[(H0.5A)2](o)0
The value of extractants is their ability to partition individual REY at different magnitudes (distribution ratios). The partitioning vs. pH curves is not linear and vary by slope and shape. This is an indicated that the stability and properties of the above complexes are different. By the time we were done with the database estimation work, we ended up with the following set of plots. This first shows the REY partitioning as a function of pH. The black dots and lines are the literature data, and the colored lines are the database predictions.
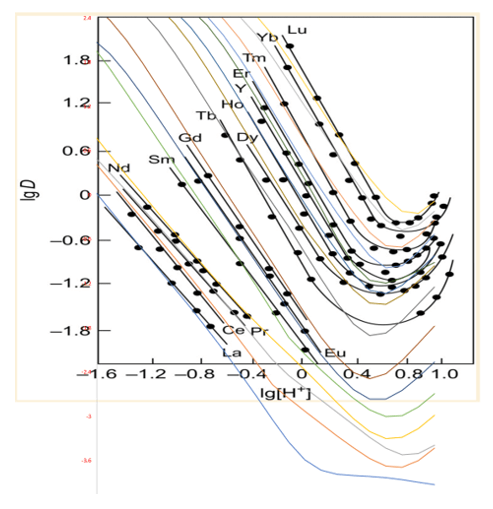
The second shows the REY partitioning as a function of HEHEHP addition. The symbols are measured data (for Y, Dy, and Nd) and the colored lines are the predictions. There seems to be disagreement between the above plot and the plot below, because the LREE curves above fall on the measured line, but are well below the reported values, especially at low HEHEHP:REY ratios.
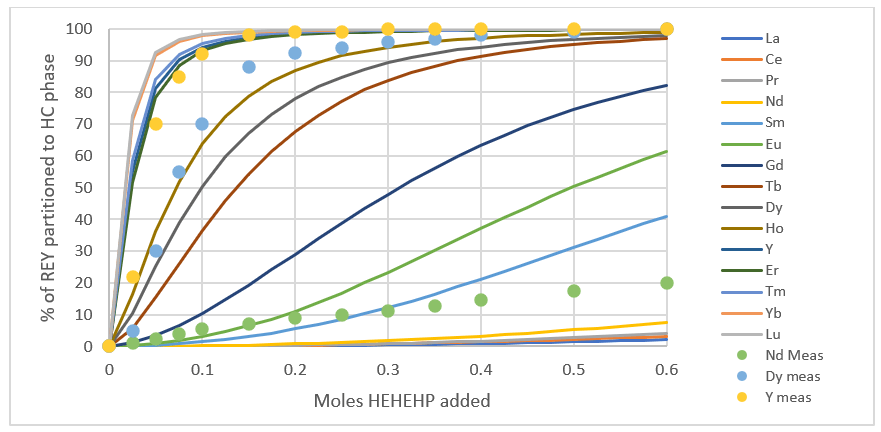
While these predictions are still only qualitative in performance (at least by OLI standards) they were good enough to use in the next step of our work which was to try it on a chemical separation simulation.
Developing a simulation study
Based on our readings, the number of solvent separation stages are counted in the hundreds. This was a process that we never tried before and one that would challenge our current software capabilities. OLI Flowsheet: ESP has an LLE separation block, but its solver was not designed to simulate this type of process. We used a series of mixer-separators instead. This presented its own challenge because the counter-current mixing in solvent extraction requires many recycle/tear streams, and the software is limited to fifteen. Lastly, we read that process problems included emulsion formation and overall separation efficiency. We use adjustable parameters to set the mass transfer and emulsion entrainment, but since this is a Process Engineering issue and not an Engineering Chemistry (OLI’s focus), these parameters are empirical.
Keeping these limitations in mind, we set up the following solvent extraction process to separates the following REY’s: Lu, Y, Gd, Eu, Nd, and La. The process it contains twenty-five separation stages set up in four sections. The first section contains five stages and removes Lu from the pregnant liquor while keeping as much of the other elements in the aqueous liquor. The second section containing eight stages removes Y, the third section also containing eight stages removes Gd and Eu and the last section containing four stages separates Nd from La. The final raffinate contains only La.
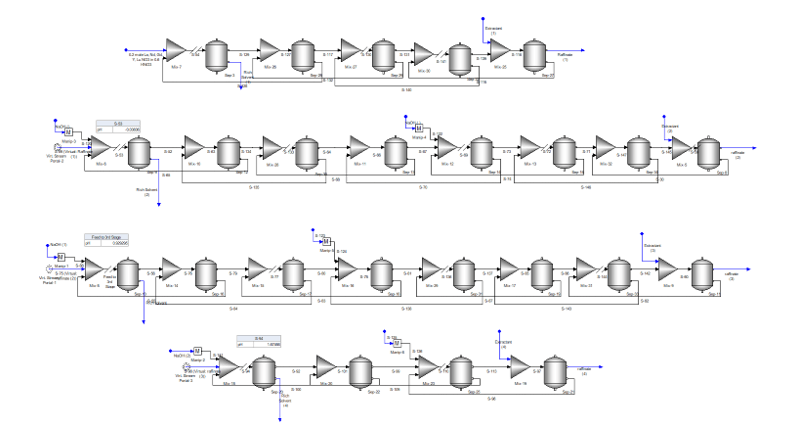
The pregnant liquor enters the process in the upper left and flows through the system until the raffinate exits at the lower right. We use virtual streams to transfer the liquor to the next step, which then makes it easier to view the process in four discrete steps. The pregnant liquor is a REY-NO3 solution at 1 pH and each REY starts out at the same concentration (a hypothetical case). Caustic (50% NaOH) and Extractant (~30 wt% / 20 mol%) in a combination of nC10 (25 mol%), nC13 (50% mol%) and nC16 (25 mole%)
The case takes about 20 minutes to run on a 5-y/o Intel I7-7700 CPU, and about half this time on the new I9 chip. The results are separation performance is shown in the table below.
| Element | Section #1 | Section #2 | Section #3 | Section #4 |
| La(+3) | 0.00% | 0.04% | 0.01% | 0.71% |
| Nd(+3) | 0.03% | 0.92% | 3.13% | 95.90% |
| Eu(+3) | 0.07% | 2.31% | 95.59% | 2.06% |
| Gd(+3) | 0.12% | 4.40% | 95.48% | 0.00% |
| Y(+3) | 3.60% | 96.06% | 0.35% | 0.00% |
| Lu(+3) | 99.49% | 0.51% | 0.00% | 0.00% |
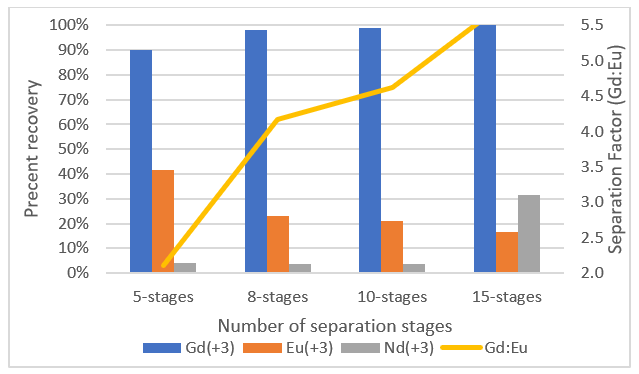
Gd and Eu partitioning behaviours were too similar to separate in eight stages, so we created a separate simulation to separate those two elements from each other. We did a comparative study of between eight and fifteen stages to see how the separation performance changed. The results are shown in the following plot.
Looking at the results and next steps
With respect to the simulation results, the good news is that the simulation worked and produced the expected results. The less good news is that we couldn’t compare it to real plant or lab data (the information is not available).
The next step is to hand out the model to clients and other interested parties. Further development will depend on user evaluation and acceptance. If you want to try the model check with your OLI representative or contact [email protected]. This is interesting engineering chemistry, and we are hoping that it makes a positive difference to those working in this field. A more comprehensive description of this work is in the ALTA 2023 (Perth, Australia) paper entitled HEHEHP database to predict rare earth solvent extraction.

