Lithium industry
Lithium is the third element of the periodic table and the first element in the alkali metal group. It has widespread applications, usually in the form of lithium carbonate, in next generation technologies, such as energy storage (lithium ion batteries (LIBs)), electric mobility and cordless devices. The worldwide demand for lithium continues to increase and this presents significant opportunity for the lithium industry, as companies race to get lithium production to market. Traditional lithium extraction from mineral ores such as spodumene, and evaporation of lithium compounds from lithium-rich brines such as those found in the salars (i.e., salt flats in Chile, Bolivia, and Argentina) have been the basis for most production schemes. Lithium recycling is also required to recapture lithium before it is lost in waste streams such as old mobile phones. The proportion of recycled lithium is forecast from currently less than 1% to 25% of the supply in 2050 [1,2]. Profitability and competitive advantage come from making lithium production either faster or less expensive, or from devising a way to obtain greater yield, purity, and production efficiency. As with other process industries, developing engineering design schemes through mathematical modeling and chemistry principles is a way to gain a competitive edge.
Process simulation deficiencies
Extracting lithium from each source (salars, lithium-bearing minerals, and to a lesser degree, lithium-bearing clays, recycled batteries, oilfield produced water, hydrothermal deposits, and seawater) employs different chemical processes. Understanding the chemical mechanisms of each process enables efficient and sustainable production, helping to maximize yields while lowering operation costs and maintaining environmental compliance. Until recently, process simulation has been able to address some, but not all aspects of optimizing lithium production schemes. What was lacking was the rigorous chemistry calculations that are critical for yield and purity optimization for the highly reactive and complex lithium chemistry. What is needed is accurate solubility calculations at high salinity, and also the simulation of the complex chemistry of double salts. These missing features made any chemistry predictions out of reach of all major flowsheet simulator systems. The industry was satisfied with “bucket chemistry,” or empirical approaches that roughly and imprecisely characterize the behavior of lithium processes.
OLI Systems electrolyte thermodynamics in lithium industry
Thermodynamic simulations are essential for achieving optimum conditions, maximizing yields, and lowering costs in lithium process techniques. OLI Systems, Inc., a global electrolyte chemical technology leader is uniquely positioned to move beyond bucket chemistry for lithium processes and supply the missing mission-critical rigorous chemistry simulation for lithium. OLI’s MSE framework [3], which is incorporated in OLI Software Platform V10, contains the requisite chemical species and calculates the thermodynamics properties that are necessary to enable science-based, data-driven, and reliable simulations. OLI Systems’ framework is sufficiently robust and extendible to deliver rigorous and accurate chemistry simulation for any complex and interactive chemistry of interest.
OLI Systems’ lithium chemistry coverage
Lithium and the components usually found together with lithium are an example of such a complex and reactive chemistry. OLI Systems first looked at the lithium + potash chemistry in the mid-2010s and quickly understood that a comprehensive model for this chemistry would be a ‘mega’ system that would encompass – to begin – Li-Mg-Na-K-Ca-SO4-CO3-Cl-OH-NO3 environments. OLI Systems assessed that this system would require literally years of study and would need a team of thermophysical modelers to collaborate on developing all the required parameters.
Can we reliably predict the behavior of lithium using OLI Software Platform?
As of 2019 with the release of OLI Software Platform V10, a major subset of OLI Systems’ lithium chemistry initiative is complete. OLI’s simulation capabilities include:
- Fundamental sulfate-chloride systems (Li-K-Mg-Ca-Na-Cl-SO4); examples are shown in Figures 1 and 2.
- Fundamental hydroxide and carbonate systems (Li-Na-CO2-OH-CO3-Cl)
- Lithium in acid environments for processing and recycling (Li-H-Cl-SO4)
- Lithium borate systems for Li production
- Systems related to Li hydrometallurgical processing, purification, and recycling (LiF, Li-Ni-Co-Mn-NH4-Cl-SO4),
- Nitrate systems related to caliche sources and thermal energy storage systems (Li-Na-Mg-Ca-NO3)
- Miscellaneous systems containing lithium chloride with silica, methanol, ethanol, and formic acid
- Systems related to battery electrolytes, such as lithium hexafluorophosphate, lithium tetrafluoroborate, and lithium perchlorate in some pure and mixed carbonates.
For the last kind of systems, the MSE framework also has the ability to model transport properties. Figure 3 is an example of electrical conductivity of lithium hexafluorophosphate in propylene carbonate. Electrical conductivity is important in battery studies because the electrolyte should have sufficiently high electrical conductivity and, at the same time, it should remain thermodynamically stable in the liquid phase in a wide temperature range in which the battery is expected to be used in practice. This can be achieved by creating appropriate formulations containing a lithium salt such as LiPF6 and highly polar organic solvents such as various alkyl carbonates.
Figure 1 shows the solubility behavior of LiCl in water as a function of temperature ranging from approximately -80 to 300 °C and indicates a good agreement of the predictions with experimental data. Besides anhydrous LiCl, four solid lithium chloride hydrates exist, with 1, 2, 3, and 5 water molecules. These salts are very soluble in water. The calculation of the thermodynamic properties of lithium chloride salts and their mixtures is a challenge to aqueous solution modeling. OLI Software Platform V10 not only predicts the solubility behavior of LiCl, but also calculates other properties of LiCl solutions (activity of water and activity coefficient of aqueous LiCl, vapor-liquid equilibria, enthalpies, heat capacities and densities of the solution).
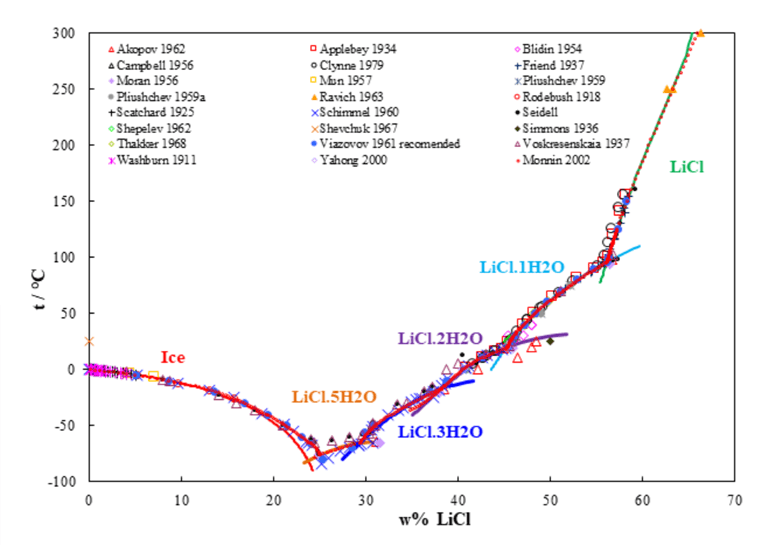
Figure 1. LiCl solubility in water as a function of temperature
Figure 2 shows that the OLI’s MSE model reliably predicts the ternary system Li2SO4-Na2SO4-H2O. From 0 °C to 100 °C, the model accurately predicts five solid phases including two different double salts, which both exist in the ternary system at 40°C. With the temperature increasing from 0 to 100 °C, the crystallization regions of solidum sulfate and the double salt (Na2SO4·Li2SO4) become larger, but the crystallization region of the double salt (3Na2SO4.Li2SO4·12H2O) disappears. The results from the MSE model provide insights and a theoretical foundation for the separation of lithium and for studying its geochemical behavior.
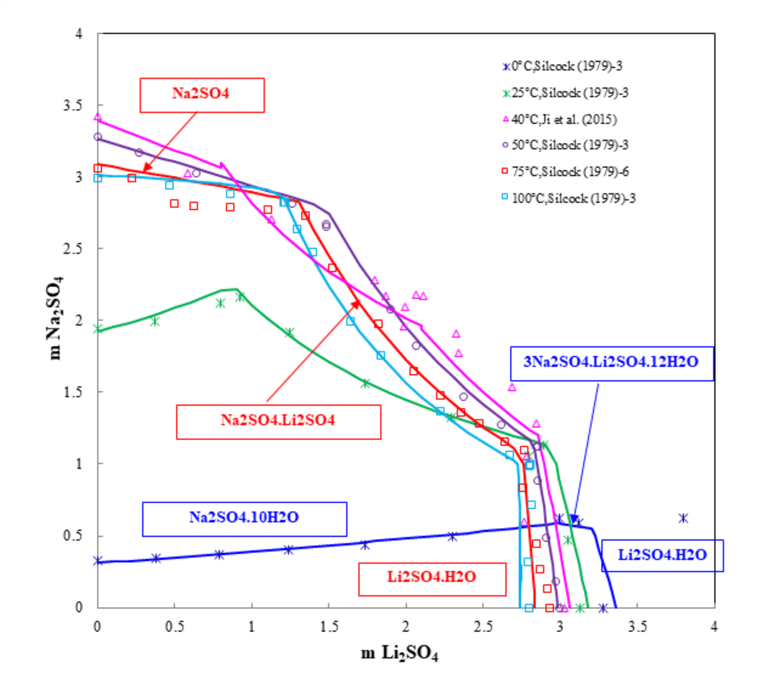
Figure 2. Ternary system Li2SO4-Na2SO4-H2O
Figure 3 shows that MSE models can predict electrical conductivity and its variation with temperature, solvent composition, and salt concentration. OLI System’s MSE model also makes it possible to predict solubility behavior of Li salts (e.g. LiPF6 and LiBF4) in pure or mixed organic carbonates and other phase equilibria, thus helping to optimize the composition and performance of the battery electrolyte. The model is calibrated by reproducing selected experimental data in binary systems within their experimental uncertainty and then it can predict the behavior of more complex battery systems.
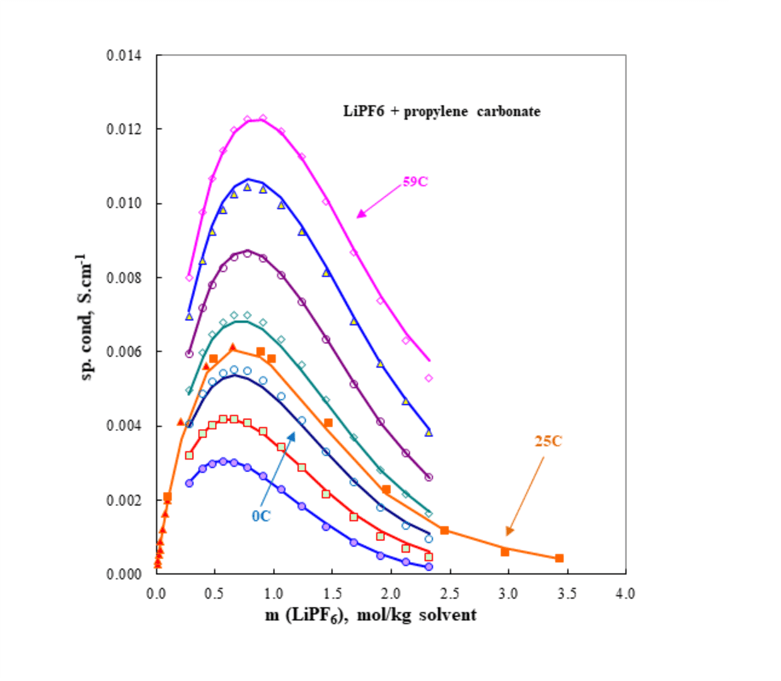
Figure 3. The electrical conductivity of LiPF6 and propylene carbonate
One of methods of extracting lithium from seawater involves using a manganese-based absorbent which has a high selectivity for the lithium ion, followed by a precipitation process. Also, mixtures of lithium sulfates and selected transition metal sulfates are important for hydrometallurgical recycling of lithium batteries. As an example, Figure 4 shows the ternary system Li2SO4-MnSO4-H2O. The prediction matches the experimental data very well.
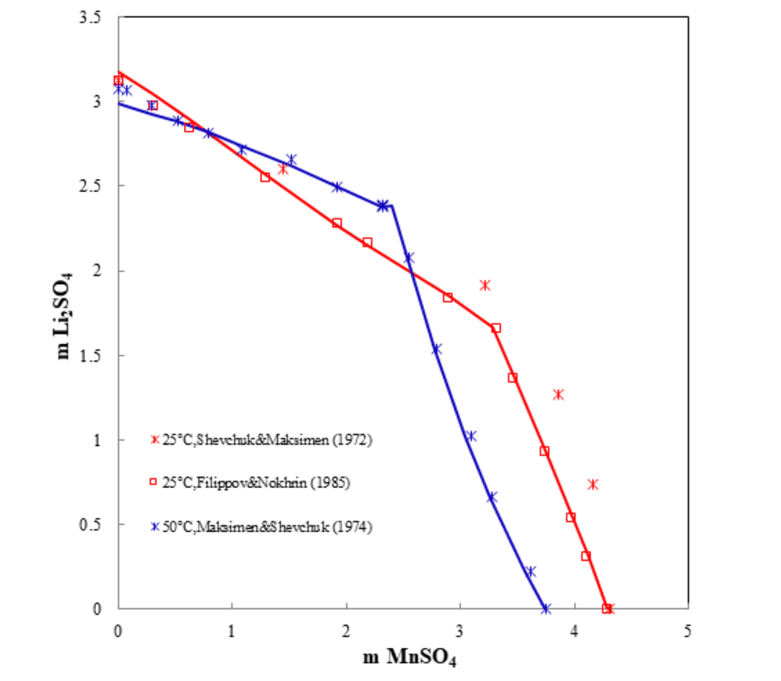
Figure 4. Ternary system Li2SO4-MnSO4-H2O
What tools are available for predicting lithium chemistry?
The implementation of lithium chemistry system provides engineers with the ability to design more efficient processes, and to predict more closely than ever before the yield and purity of their products. Measurable quantities like pH, conductivity, concentrations, and TDS, are now firmly rooted in a fundamental physico-chemical basis.
OLI Systems’ simulation packages, which is based on the MSE model, is available in OLI Studio V10 and OLI Flowsheet ESP V10.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- B.K. Reck, T.E. Graedel, Challenges in metal recycling, Science 337 (6095) (2012), 690–695.
- G.Angerer, Rohstoffe für Zukunftstechnologien: Einfluss des branchenspezifischen Rohstoffbedarfs in rohstoffintensiven Zukunftstechnologien auf die zukünftige Rohstoffnachfrage, Fraunhofer-IRB-Verl., StuttgartISBN: 3-8167-7957-3, 2009.
- Wang P., Anderko A., Young R. D., “A Speciation – Based Model for Mixed – Solvent Electrolyte Systems”, Fluid Phase Equilibria, 203, (1-2), 141-176, 2002.