The demand for rare earth elements (REEs), a group of 17 metals naturally found in the environment, including 15 lanthanides, scandium, and yttrium, has significantly increased due to their indispensable uses in numerous advanced materials for electronics, energy, transportation, defense, and communications applications. However, due to the extensive use of high-tech applications in our daily lives and the depletion of their primary ores and environmental hazards related to their mining, REE’s recovery from secondary sources is needed today. Therefore, REEs have now attracted attention to policymakers and scientists to develop novel recovery technologies for materials’ supply sustainability. Recycling electronic scrap or e-waste, which contains electronic displays, speakers, cell phones, motors, hard disk drives (HDDs), voice coil actuators, and other materials, is a significant source of rare earth metals. Although traditional recycling routes for some electronic scrap emphasize the recovery of silver and gold, value can also be attained by recovering rare earth elements from these unique feed streams.
As of 2016, 20% of e-waste was recycled worldwide in a documented, proper manner, but the rest is either dumped or shipped to other companies where it is recycled in a crude manner. However, REEs are not recovered to any significant extent. In fact, less than 5% of rare metals were recycled from e-waste as of 2019. Although there is an increased interest in recycling research, few recycling methods for REEs have reached the commercial scale, and most secondary sources of REEs are not being recycled. In recent years, several literature sources have outlined novel recycling technologies along with potential REE recycling feedstocks as the demand for rare earth oxides is predicted to increase fivefold by 2030. The recovery of REEs from e-waste could be an effective way to meet future needs and bolster economic growth.
Increasing sustainability through REE recycling
The challenge to e-waste recycling germinates from the fact that manual separation and shredding are essential in the initial stages of the process cycle which are labor and equipment intensive as it requires disassembly or dismantling, physical separation, and size reduction. In the subsequent steps, chemical processing further adds complexity to the challenge as electronic wastes contain oxides, metals, non-metals, and polymeric materials, rendering effective separation and recovery essential. However, all these challenges are outweighed by the benefits.
REE recycling has significant advantages over the mining of REEs, including savings in energy, water, and chemical consumption, along with a substantial reduction of emissions, effluents, and solid waste generation resulting from the extraction and processing of rare earth ores. REE recyclates do not contain radioactive thorium and uranium, unlike the primary mined rare-earth ores.
Therefore, radioactive tailing stockpiles and mining health problems can be, at least partially, avoided. There are also possible benefits from avoiding land allocation for the mine and radioactive waste streams and transportation. Furthermore, recycling helps address the so called “balance problem”, namely the fact that certain REEs with high level of demand (e.g. europium, dysprosium) are present in small quantities in REE ores along with other REEs that have low demand. This means that in order to meet the demand for the former, the latter are produced in excess and are stockpiled. If more recycled metals are recovered for value, less mining is required, and waste is minimized. In a nutshell, e-waste recycling for REEs lays a pathway to meet future demands in a more environmentally sustainable and efficient manner.
Outlining a novel method for the recovery of REE from electronic scrap
In the recent years, several research efforts have outlined processes for the efficient recovery of REEs from e-wastes through bioleaching, biosorption, siderophores, electrochemical, nanomaterials, cryo-milling, supercritical CO2, hydrometallurgical, and pyrometallurgical based approaches. In one such effort, Lister et al. [1] has proposed a novel hydrometallurgy based technology to recover rare earth elements from mixed steel Nd-Fe-B alloy material, a feed from shredded HDDs, using HCl as a re-usable extraction medium. In the first step, the Nd-Fe-B alloy material mixture was selectively leached using 1m HCl with pH ~ 0. Following the dissolution, REEs were precipitated using sodium sulfate (Na2SO4) as the sodium double salt (Na2REE2(SO4)4).
Subsequently, the resultant double salt is converted to rare earth hydroxide (REE(OH)3) using NaOH. The OLI software platform V10 has played an integral part in the development of this technology as it provided the modeling prowess necessary to decide on the separation technique to precipitate out the REE from the solution as the double salt and later recover it as purest REE(OH)3 form.
How OLI’s thermodynamic framework has played a central role
The MSE thermodynamic framework in the OLI software platform V10 can accurately estimate solubilities of a wide variety rare earth containing multi-component systems including chlorides, sulfates, fluorides, hydroxides, carbonates, citrates, tartrates, and oxalates over wide ranges of process conditions such as pH and temperature [2, 3]. The model parameters for REE-containing systems, including the REE2(SO4)3 [2], were developed ensuring that the model matches the available experimental data for solid–liquid equilibria, vapor–liquid equilibria, and caloric properties.
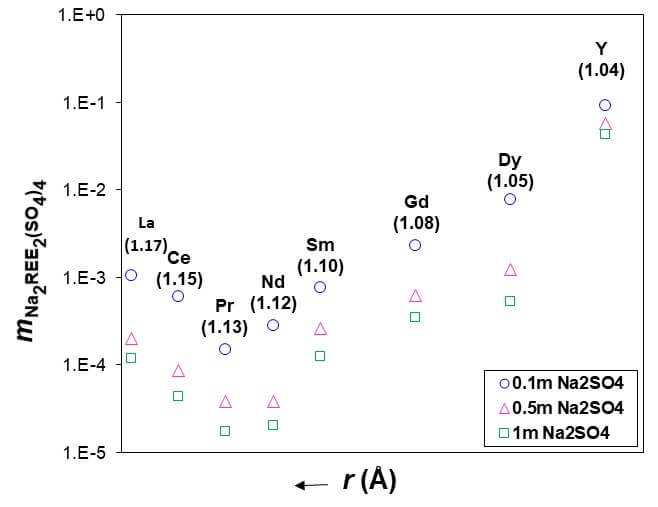
Subsequently, the model was used to calculate the solubility of REE— sodium sulfate double salts for many of which no experimental data are available [3]. Figure 1 presents the solubilities of different Na2REE2(SO4)4 salts at 25 °C for different Na2SO4 concentrations as a function of crystal cationic radii of REEs. The solubilities manifest a pronounced minimum in the solubility for Pr. Starting with Nd, the solubility increases as a function of crystalline radius and becomes substantially higher for heavy REEs. This provides a thermodynamic basis for the amounts of the solids that are expected to precipitate during the separation process as Na-REE double sulfates. This fundamental thermodynamic analysis has played a key role in developing hydrometallurgical process for the recovery of rare earth elements from hard disk drives after HCl leaching.
In fact, the recovery of rare earth elements in the process is consistent with amounts predicted by the MSE (Mixed-Solvent Electrolyte) framework for precipitated double salts. The predicted recovery can be compared with the XRF data. The comparison of the model and experimental data presented in Figure 2 shows the Nd showed the most significant recovery followed by Pr. The other REEs, such as Dy showed intermediate recovery while La and Y showed poor recovery. The lower recovery was presumably due to the lower starting concentrations close to the solubility limit for the double salt. There is a little discrepancy between measured and calculated results for Dy where the MSE model predicted no recovery. This may be due the co-precipitation of Dy and Nd owing to the overwhelmingly larger amount of Nd in the samples. It is well known that rare earth salts have a strong propensity to form solid solutions containing two or more rare earth elements. This has been observed for multiple classes of compounds, including REE oxides, chlorides, nitrates, and cuprates. Although the formation of solid solutions has not been reported for REE—Na double sulfate salts—it can be reasonably presumed that solid solutions are also possible for this class of compounds. If this is the case, then a relatively small amount of Dy may be incorporated into a Nd-dominated double salt, thus leading to the recovery of Dy together with Nd.
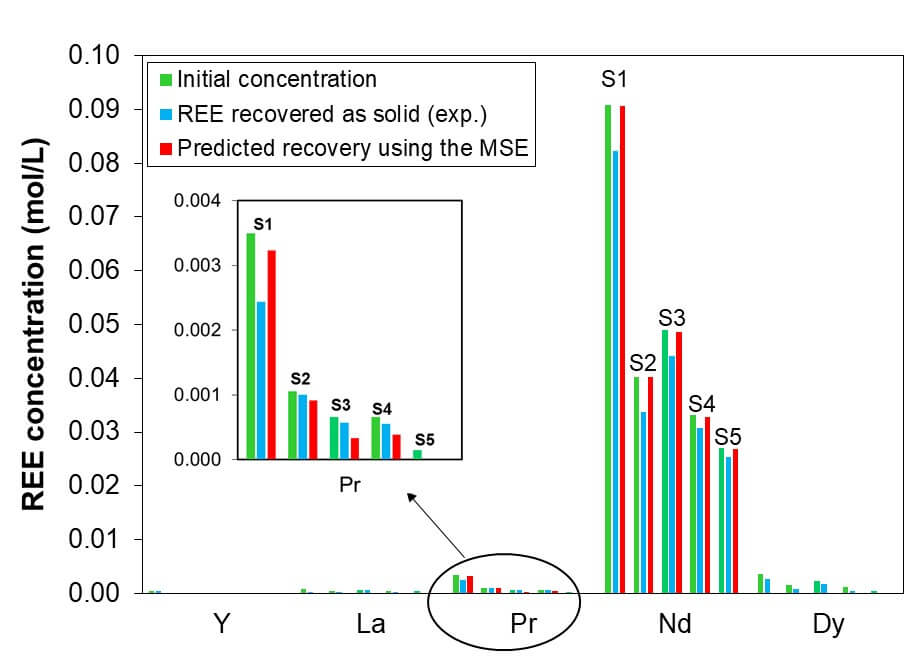
The green bars represent the initial concentration of REEs in the leaching solution, while the blue and red bars give the experimental and predicted recovery, respectively. The symbols S1–S5 denotes samples 1–5.
What tools are available for predicting REE properties?
The OLI System’s thermodynamic property package, which is based on the MSE model, is available in OLI Studio V10 and OLI Flowsheet ESP V10. For more updates on this project and the OLI System’s thermodynamic property package, its components and applicability, follow my ResearchGate page https://www.researchgate.net/project/A-broad-based-study-of-thermodynamic-properties-of-rare-earth-element-containing-compounds-for-industrial-separation-purposes.
Contact OLI for more information or to schedule a meeting with an OLI expert.
References
- Lister, T.E., Meagher, M., Strauss, M.L., Diaz, L.A., Rollins, H.W., Das, G., Lencka, M.M., Anderko, A., Riman, R.E. & Navrotsky, A.. “Recovery of Rare Earth Elements from Recycled Hard Disk Drive Mixed Steel and Magnet Scrap.” In Rare Metal Technology 2021,139-154. Springer International Publishing, 2021
- Das, G., Lencka, M. M., Eslamimanesh, A., Wang, P., Anderko, A., Riman, R. E., & Navrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. The Journal of Chemical Thermodynamics, 2019, 131, 49-79
- Das, G., Lencka, M. M., Eslamimanesh, A., Anderko, A., & Riman, R. E. Rare-earth elements in aqueous chloride systems: Thermodynamic modeling of binary and multicomponent systems in wide concentration ranges. Fluid Phase Equilibria, 2017, 452, 16-57

