Simulating nuclear waste disposal applications in Belgium and Sweden with the OLI Software Platform
The Nuclear Waste Context
High Level Waste (HLW) contains many of the fission products and transuranic elements generated in the reactor core and is the type of nuclear waste with the highest activity. HLW accounts for over 95% of the total radioactivity produced in the nuclear power process. In other words, while most nuclear waste is low-level and intermediate-level waste, such as protective clothing and equipment that have been contaminated with radiation, the majority of the radioactivity produced from the nuclear power generation process comes from high-level waste.
The challenge of making nuclear power safer doesn’t end after the power has been generated. Nuclear fuel remains dangerously radioactive for thousands of years after it is no longer useful in a commercial reactor. The resulting waste disposal problem has become a major challenge for policymakers.
This article analyzes the asset integrity challenges to nuclear waste storage containers in two European countries.
Nuclear Waste Disposal in Belgium
Belgium is considering the disposal of High-Level Nuclear Waste (HLNW) using supercontainers to encapsulate the waste that would then be stored permanently (for 100,000 to 1,000,000 years) in clay repositories. It is assumed that the principal threat to container integrity is corrosion of the carbon steel overpack in contact with Portland cement concrete, which is situated between cylindrical overpack and outer, cylindrical stainless steel envelope (Figure 1).
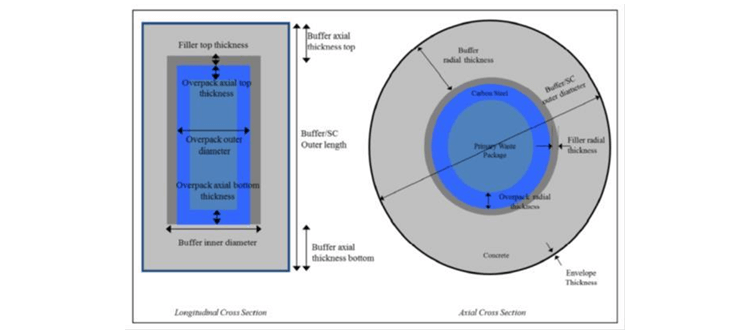
Figure 1. A longitudinal cross-section through the super container illustrating the dimensions of the various components. After ONDRAF-NIRAS [1].
It is evident that no firm experimental data are currently available to estimate the probability of failure over the extended storage time (100,000 years). Accordingly, the only possibility to estimate propagation of corrosion damage (probability of the failure of the constriction) is to develop a realistic model based on the application of natural laws. This model has to allow users to estimate the corrosion potential, rates of general corrosion of the overpack, probability of pit nucleation, hydrogen pressure, water saturation, and the porosities of the rust and the concrete as corrosion proceeds along the corrosion evolutionary path. Thus temperature on the overpack surface is determined by the cooling of the overpack filled with vitrified high-level waste (VHLW), mixed oxide (MOX) spent fuel (MOX-50) or uranium oxide (UOX) spent fuel (UNE-55). In turn, the corrosion product occupies 2 – 6.5 times the volume of the iron from which it forms, the development of the outer layer in the confined space between the carbon steel overpack and the concrete results in the compression of the outer layer and in a concomitant decrease in the porosity. This effect can be important, because if the porosity decreases to a sufficiently low value, the growth of the outer layer ceases and future corrosion may be prevented. From the mathematical point of view the application of the developed model reduces to the solution of the system of charge, mass and heat transfer equations along with mechanical equations describing stresses in the system.
However, the prediction of such model can be reliable only if the sufficiently reliable kinetic and thermodynamic properties are established. OLI software platforms were used for establishing analytical relations for these properties among which there are partial particular current densities, diffusion coefficients, activities of ions, viscosity, density, etc. [2 – 6]. After that these relations were used by the numerical solutions of mass and heat transfer equations.
However, the most non trivial application of OLI software platforms was the estimation of the properties of mixtures (cement + water) as a function of the mass of added water (wetness of the mixture).
For example, for the particular cement composition (altogether 10 different cements were investigated) shown below (numbers under symbol of each component are mole % in the cement mixture contained totally 100 mole of different compounds)
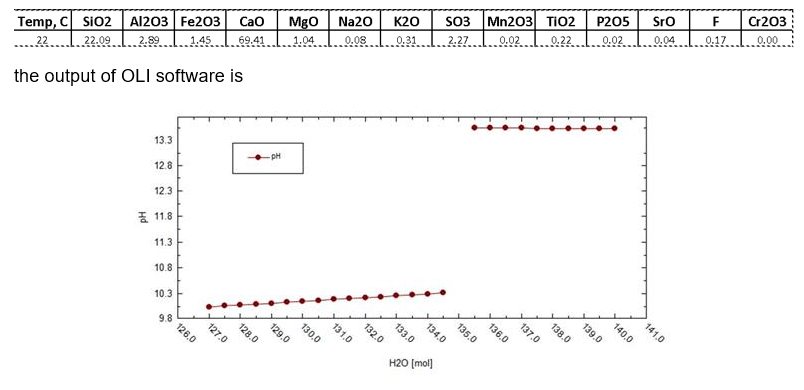
When the amount of added water is less than 127 mole there is no liquid phase in the system.
It must be noted that such calculations can be performed only by using the mixed solvent electrolyte model (MSE) and, in principle, cannot be performed by using the aqueous model [3, 4]. In accordance with our knowledge OLI software is the only available commercial software that can be used for such calculations.
By using all kinetic and thermodynamic parameters obtained by OLI models and software the solution of the system of charge, mass and heat transfer equations along with mechanical equations was solved. The example of results of calculations is presented on Figure 2 [7].
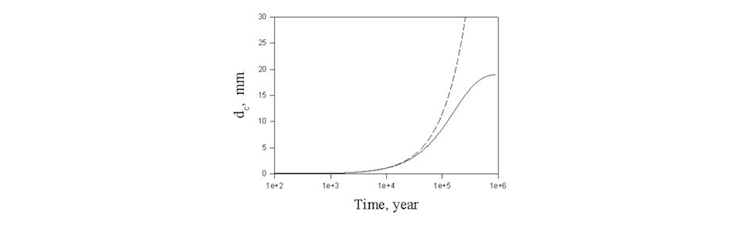
Figure 2. Thickness of the corroded layer as the function of time (solid line) and by neglecting the changes of the porosity of the rust (dash lines).
It means that under normal conditions the thickness of corroded layer is less than the design thickness of the carbon steel overpack (30 mm) even after 1000000 years.
Waste disposal in Sweden
Figure 2 shows schematically the canister located in the repository that is being developed in Sweden. It is planned that the canister will be emplaced at the depth of several hundred meters (where there is no oxygen in the groundwater) [8]. The canisters will be encased in compacted bentonite, which will be used as a buffer. The main role of the buffer is to prevent the surrounding environment from penetrating the canister and hence from releasing highly radioactive species in the waste, should the canister be ruptured. However, the buffer also protects the copper surface of the canister from corrosion.
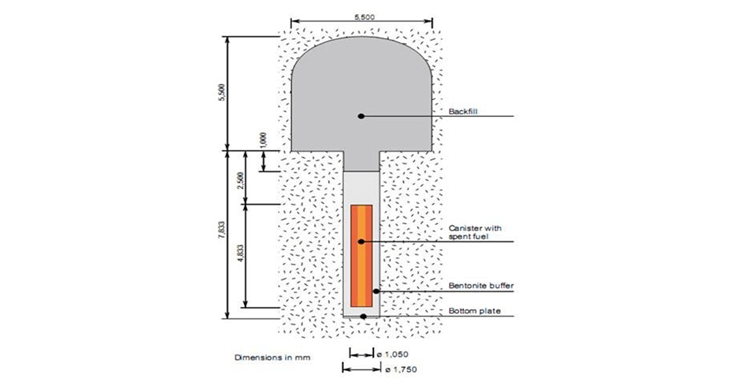
Figure 2: Schematic of a copper canister emplaced concentrically with the bentonite buffer located in a cylindrical hole in the floor of a tunnel in the repository [8].
The OLI software was specifically used for the estimation of the maximum possible corrosion current density (and accordingly the maximum thickness of corroded layer, dcorr) in the repository during design service life (100000 years). Thus, it is assumed that the rate of corrosion is limited by the transport of HS– ions to the copper canister, where the electrochemical reaction:
2Cu + HS– → Cu2S +H+ + 2e–
takes place. Flux density of HS– in the porous media is proportional to the diffusion coefficient of HS–, which was calculated as a function of temperature and solution composition by using the OLI software. By using this calculated value of DHS- the solution of the system of charge, mass and heat transfer equations along with mechanical equations was solved. The results of calculations are presented on Figure 3.
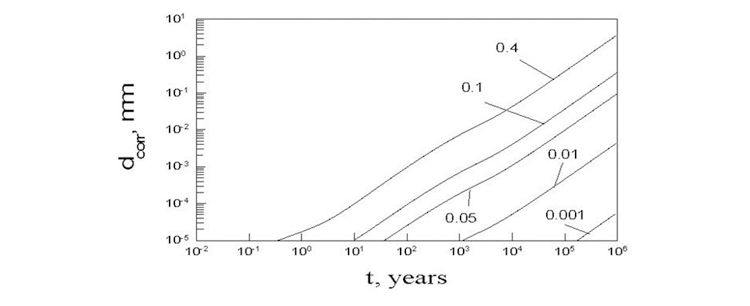
Figure 3. Thickness of corroded layer as a function of time for different initial values of the porosity of the bentonite. The hump in the figure arises from the variation at the canister surface.
Accordingly, we conclude that under normal conditions the thickness of corroded layer is much less than the design thickness of the copper container (50 mm). Of course, under some catastrophic conditions, for example, by increasing concentration of HS– in ground water by two orders of magnitude the copper container can be destroyed.
As demonstrated above, the OLI software makes it possible to model the integrity of complicated industrial systems such as nuclear waste repositories, where the prediction of integrity must cover at least 100000 years, by providing reliable thermodynamic and kinetic properties (in particular, current densities, diffusion coefficients, pH, activities of ions, viscosity, density, prediction of speciation in the solution and even prediction of the existence of liquid phase in the system).
For more information on the OLI Software Platform visit https://www.olisystems.com/oli-platform-v10 ; for more information on the OLI Studio: Corrosion Analyzer, visit
https://www.olisystems.com/oli-studio-corrosion-analyzer.
To speak to an OLI technical or sales specialist please register your interest here https://www.olisystems.com/contact or email [email protected]
References:
- Design and construction of the supercontainer for category C waste”, ONDRAF-NIRAS, Brussels, Belgium, Report NIROND-TR 2017-11 E V2, 2017.
- Anderko, “Modeling of Aqueous Corrosion,” in Shreir’s Corrosion, ed. T.J.A. Richardson (Amsterdam, Netherlands: Elsevier, 2010), pp. 1585 – 1629.
- Wang, A. Anderko and R.D. Young, “A Speciation-Based Model for Mixed-Solvent Electrolyte Systems” Fluid Phase Equilibria, 203 (2002) 141-176.
- Wang, R.D. Springer, A. Anderko and R.D. Young, “Modeling Phase Equilibria and Speciation in Mixed-Solvent Electrolyte Systems” Fluid Phase Equilibria, 222-223 (2004) 11-17.
- Wang and A. Anderko, “, “Modeling Self-Diffusion in Mixed-Solvent Electrolyte Solutions” Ind. Eng. Chem. Res., 42, 3495 – 3504 (2003).
- Wang, A. Anderko, R. D. Young, “Modeling Viscosity of Concentrated and Mixed-Solvent Electrolyte Systems” Fluid Phase Equilibria., 226, 71–82 (2004).
- R. Engelhardt, B. Kursten, and D.D. Macdonald, Corrosion of Carbon Steel in Physically Constrained Locations in High Level Nuclear Waste Isolation Systems, Corros. Sci., 177, 108974 (2020).
- Swedish Government Official Reports. The Swedish National Council for Nuclear Waste’s Review of the Swedish Nuclear Fuel and Waste Management Co’s (SKB’S) RD&D. Programme 2010. Translation of SOU 2011:50.