OLI has seen increased interest in gas scrubbing since carbon capture achieved headline status. CO2 is one of several acids found in natural and combustion gases, and while it is a hot topic, we thought it might be useful to begin our blog series on gas scrubbing with H2S, a molecule that’s been scrubbed out of natural gas for decades.
H2S is poisonous and corrosive and must be removed to ppm levels before the gas is sent to the sales line. The sweetening process absorbs H2S by contacting the natural gas with a sweetener chemical (an alkaline chemical or base). The chemical is usually an amine and reacts with dissolved H2S to produce HS-1 (bisulfide). This drives H2S(g) to dissolve further into the scrubbing solution, thereby removing it from the vapor.
While this acid-base reaction absorption is simple, it is made a bit more complex by factors like temperature, pressure, and gas/solution composition. We wanted to explore these factors from a thermodynamic perspective, to see how H2S absorption efficiency is affected by running at low and high temperatures, low and high pressures, and low to high base:H2S ratio. This analysis is within our software’s strength, enabling us to gain some insight into this aspect of gas scrubbing. I’ve mentioned before that OLI Systems are a group of Chemical Engineers and Scientists and are engaged in developing Engineering Chemistry solutions. So, this blog discusses gas scrubbing from that perspective. We can rely on the scrubber designers and operators to optimize process unit itself.
General explanation of the reaction
Gas scrubbing relies on the basic chemical mechanisms learned in secondary school; this number of moles of base is needed to neutralize this number of moles of acid. When the reaction time is long and the contact area is high (area), the reaction reaches equilibrium. When the process is at ambient temperature and pressure, the extent of the reaction matches the equilibrium constants (K’s) reported in textbooks. When the scrubber solution starts with only water and base (no reaction products), the reaction reaches its maximum potential.
The challenge is when conditions aren’t ideal or ambient. This affects overall scrubbing efficiency So, here are the questions we asked. What improves or diminishes, at least thermodynamically, gas sweetening extent, and how can conditions be changed to achieve maximum potential?
A few equations
Gas scrubbing can be written in two steps, dissolving H2S into the water.
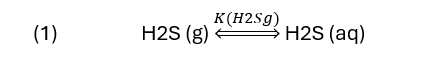
and converting H2S to HS-1. If a base like NaOH is used, then the reaction is.
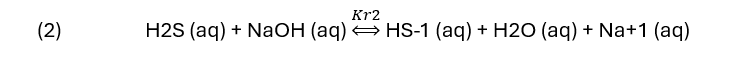
For a carbonate base (Benfield)
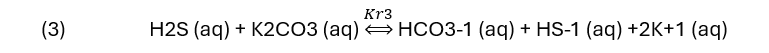
Or, for amine sweetening, the reaction is.
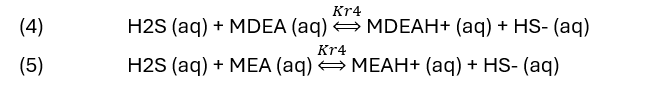
Each reaction shows H2S (aq) being converted to HS-1 (aq) using a base. The K-values in reactions (2) to (5) are in fact, several equilibrium constants shown as a single constant. Their specific name is not important to this blog other than that they will change when the reaction temperature changes.
Reactions (2) to (5) is written with H2S(aq) as a reactant, but that really isn’t measured, H2S(g) is what gets measured. Therefore, it will be easier to evaluate the sweetening extent if we replace H2S(aq) with H2S (g). To convert H2S(aq) to H2S(g) we use reaction (1) and the equilibrium equation associated with it.
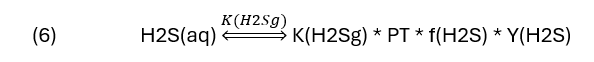
The terms in equation (6) are as follows: K(H2Sg) is the solubility constant of H2S between the gas and water. It is similar to Henry’s constant. PT is total pressure, Y(H2S) is the concentration of H2S in the vapor phase (ppmV for example). The last term is the fugacity coefficient, it’s an important factor in H2S solubility when the pressure is ~20 atm and higher. Fugacity coefficients are important and really deserve its own discussion, so we’ll gloss over it in this blog knowing full well that it really needs to be explained.
The neutralization reaction is therefore:
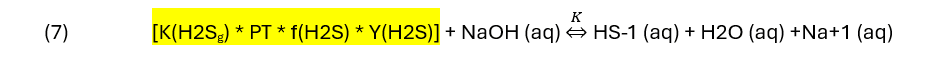
At a glance, you can see that if any of the values on the left side increases, the reaction proceeds from left to right. For the remaining agents, the equations are.
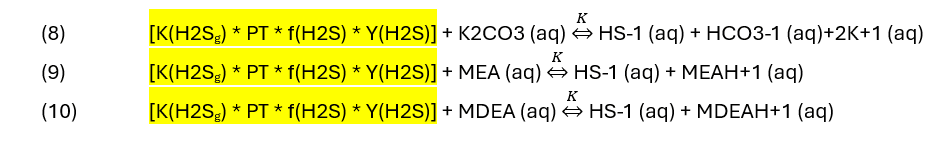
It’s clear that by increasing the concentration of base or by increasing pressure (PT), more HS-1 (aq) forms. But the reverse reaction can also occur. As the reaction products, HS-1, HCO3-1, MEAH+1 and MDEAH+1 increase, they can also convert back to H2S, MEA, K2CO3, and MDEA. Thus, in practice, the products are removed so that sweetening process is kept at the maximum extent. This is the purpose of the regeneration unit, to remove the HS(-1) and to convert the HCO3-1, MEAH+1 and MDEAH+1 back to MEA, K2CO3, or MDEA. Equations (1) to (10) therefore helps us understand the relationship between the H2S (acid) and the sweeteners (base), their reaction products and why a continuous process of absorption – regeneration is essential for effective sweetening.
Applying the equations above to see how they affect sweetening extent.
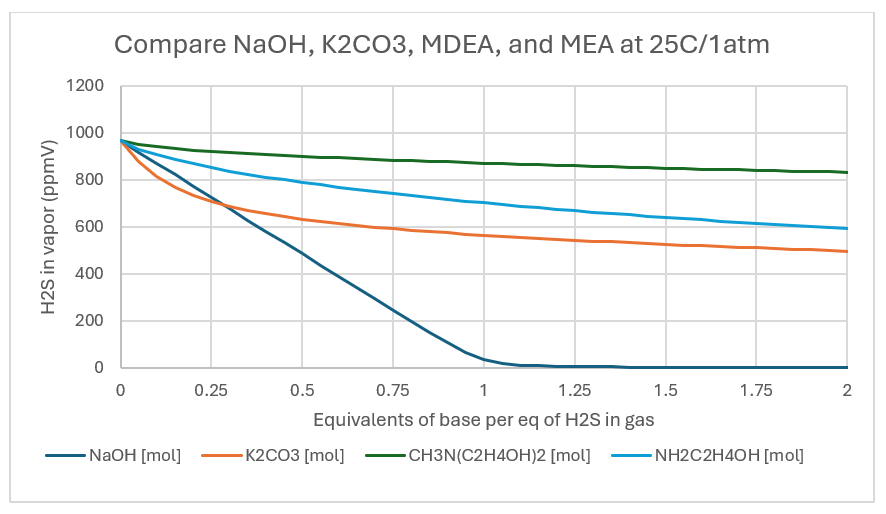
The extent of reaction is shown in the following set of plots. We simulate a batch reaction between 1kg H2O containing a base and 1 mole of H2S(g) in 1000 moles of N2 (1000ppmV). T and P are ambient (25C/1atm), and the base is added between 0:1 and a 2:1 mole ratio of base:H2S. The x-axis in the plots is that mol:mol ratio value. The y-axis is the H2S remaining in the gas (ppmV). Since this is a batch example the gas and liquid remain in the same container, and only the amount of base changes. Thus, there is buildup of reaction products as we add the base.
NaOH, a strong base consumes all the H2S once the NaOH:H2S reaches 1:1 ratio. The H2S line line is straight, indication that the HS-1 reaction product didn’t limit the extent of reaction. At a NaOH:H2S ratio of 1:1, 96% of the H2S is removed from the vapor. By comparison, the K2CO3, MDEA, and MEA lines are not linear. All three are weak bases, and they neutralize H2S less readily than hydroxide. The curvature indicates a limiting reaction extent, though the detailed cause cannot be explained by this plot alone. At the 1:1 base:H2S ratio, K2CO3 reduces the H2S(g) by about 44%, MDEA and MEA reduces H2S(g) by 13% and 30%, respectively. If the strength of the base was the only consideration, then it would be clear that NaOH is the chemical of choice for gas scrubbing.
The second plot shows the impact of raising the pressure to 20 atm on scrubbing extent. As described by reactions (7) to (10), raising pressure increases the H2S (aq) solubility and that shifts the reaction to the right. At 1:1 base:H2S mole ratio, K2CO3 removes 75%, MDEA removes 37% and MEA removes 66%.
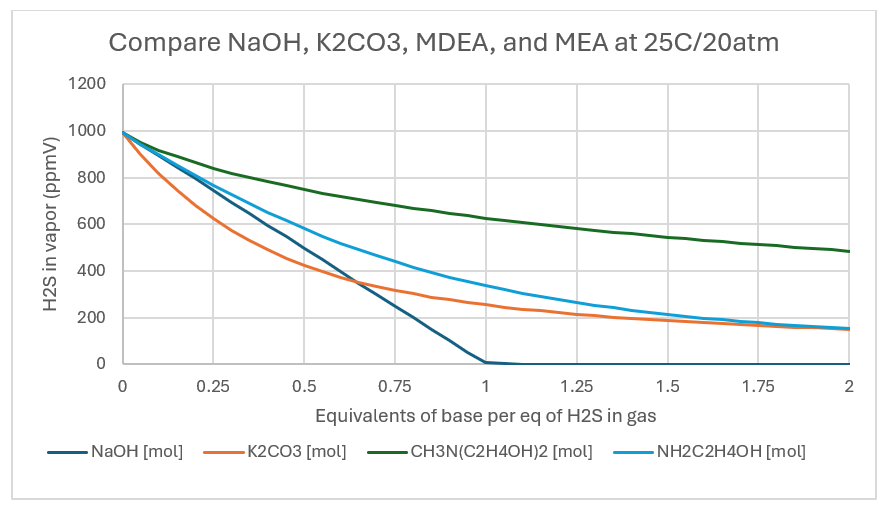
If the temperature is raised to 50C, then the K-values (equilibrium constants) change. NaOH consumes all the H2S and is unaffected by the increased temperature. K2CO3 removes 74% of H2S, MDEA removes 25% and MEA removes 47% when the base:H2S mole ratio is 1:1.
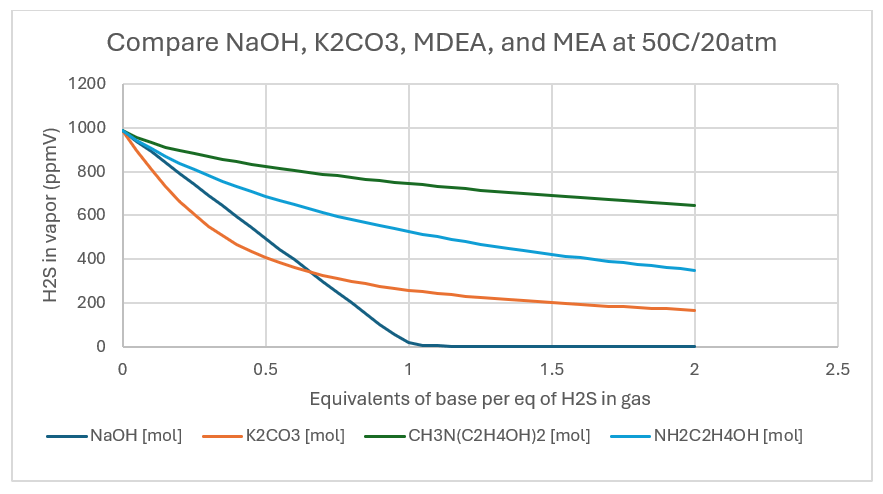
It does however, reduce the sweetening sextent of MDEA and MEA.
A glimpse into the process
The above discussion illustrates at a high level how factors like temperature, pressure and base:H2S ratio affect the sweetening extent. Some of these factors can be kept as rules of thumb, but the challenge is aggregating them into the best scrubbing solution composition and scrubber conditions. The significance of these factors on plant performance has been known for decades, which is why process simulation software and electrolyte thermodynamic models are useful for this purpose; there are simply too many factors to juggle when looking for an optimum condition. The process below is a natural gas scrubber unit created using OLI Flowsheet ESP. The scrubber uses a base to remove H2S from natural gas.
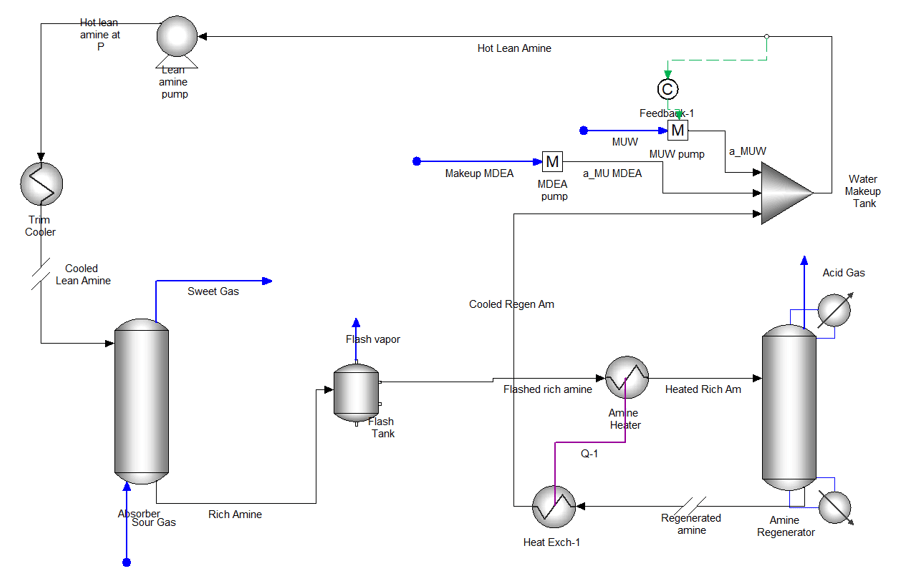
To illustrate the impact of thermodynamic and electrolyte properties on H2S removal, we simulate the effect of temperature, pressure, and base:H2S ratio on scrubber performance. Only the scrubber unit is simulated to simplify the process, meaning that fresh scrubbing solution is added to the column. By simulating the absorber column only, we can focus on the chemical properties, and not the impact of the regeneration column performance. The gas flows at a constant 1000 moles/hr N2 and 1 mole/hr H2S (1000 ppmV). The scrubber solution flows at 3 kg/hr H2O plus between 0 and 1 moles/hr of the base. We varied temperature between 10 and 50C, pressure between 1 and 20 atm, and base:H2S ratio between 0 to 1 mol:mol. We use the built-in Sensitivity to vary the three independent parameters.
The plots below are for the absorption column output at 25C/1atm. When the base:H2S ratio is 1:1, the H2S removal is 64%. 13%, and 34%, for K2CO3, MDEA, and MEA, respectively. This compares to 44%, 13%, and 30% for the same list when the sweetening was done in a single stage.
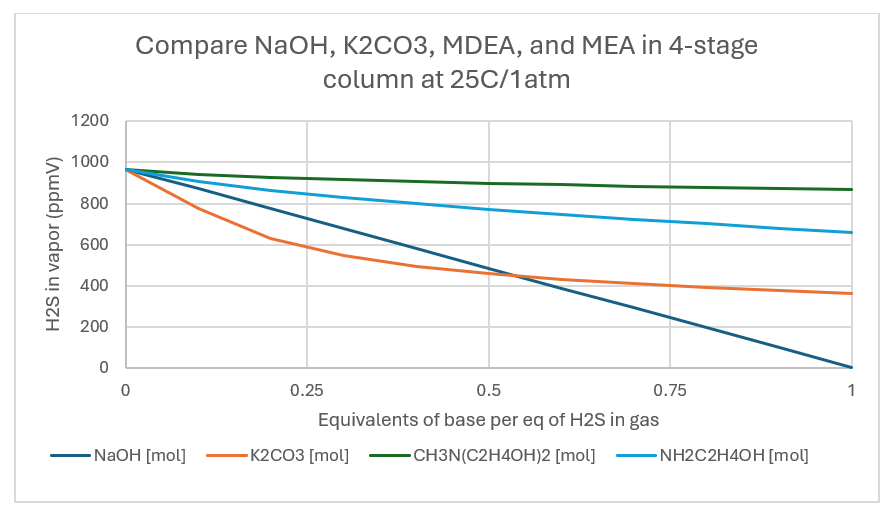
When the pressure is raised to 20 atm, the removal at a 1:1 base:H2S ratio is 94%, 45%, and 84% for K2CO3, MDEA, and MEA. This is a significant improvement compared to the 1 atm column.
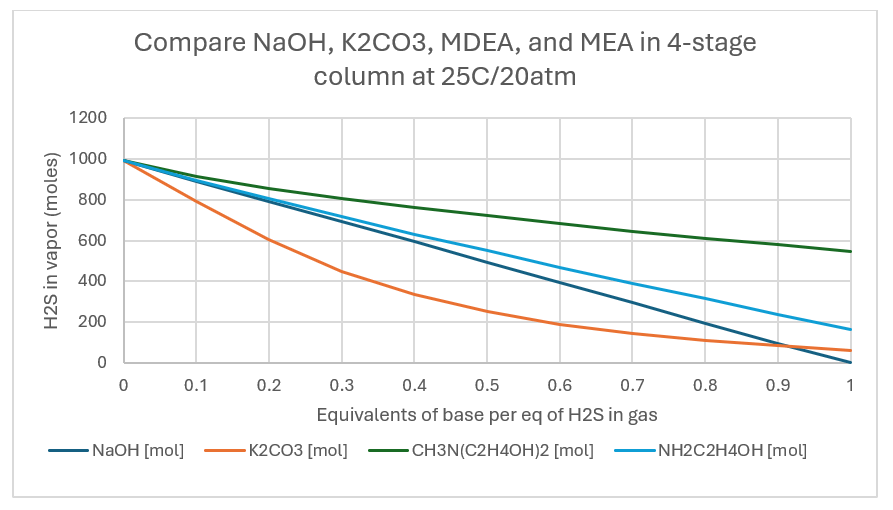
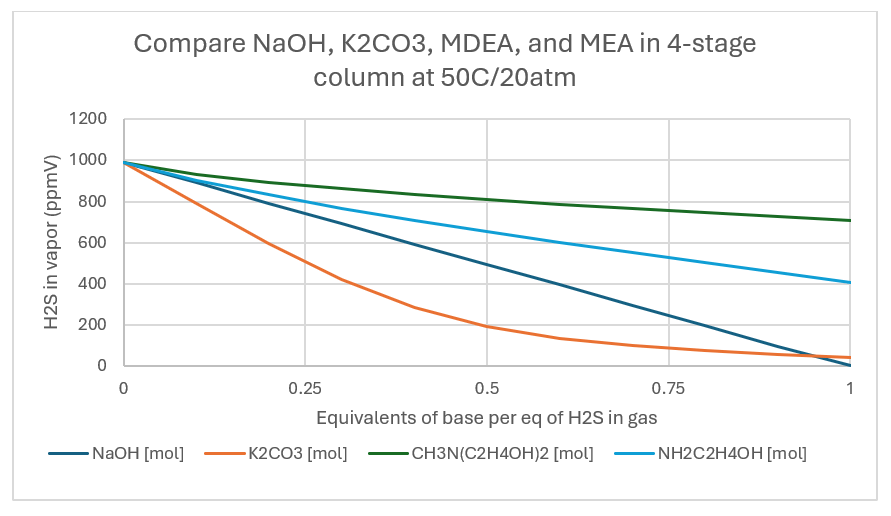
When the temperature is raised to 50C and pressure kept at 20atm, the removal is 96%, 29%, and 59% for K2CO3, MDEA, and MEA. The sweetening extent doesn’t change for K2CO3 but decreases for MDEA and MEA.
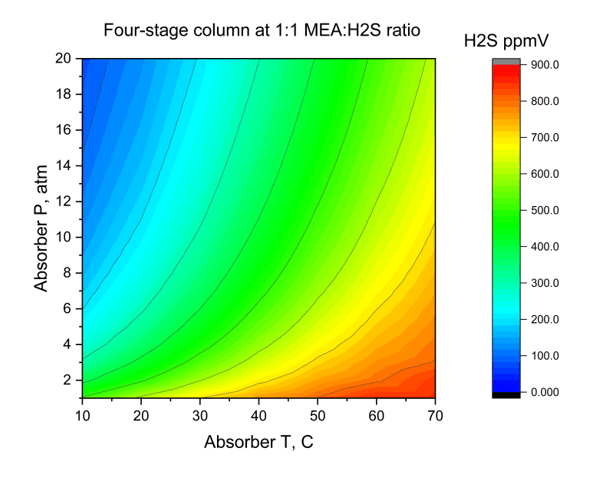
The last plot is a survey over absorber temperature and pressure for when the ratio of MEA:H2S is 1:1 mol/mol. The x-axis is the absorber temperature, the y-axis is the absorber pressure and the color contour is the remaining H2S in the vapor (ppmV). The plot confirms what we recognized above, that higher pressures and lower temperatures are better, at least thermodynamically for MEA to sweeten H2S from the gas.
Conclusion:
The role of equilibrium thermodynamics in this discussion is to show the underlying drivers of gas sweetening. Thermodynamics lets us see what is achievable and what is not, based on the conditions used. For instance, it doesn’t matter how efficient a column is, if the base used is too weak to react with the acid gas, sweetening targets will never be achieved. Or, if the pressure is low, then the dissolved H2S concentration is too low to drive the reaction to the products without an excessive number of separation stages or a larger base:H2S ratio.
Whether for H2S, CO2, SOx, NOx, or other regulated components, designing and operating gas scrubbers means understanding and managing (or in some cases exploiting) the thermophysical properties of the scrubber solution. In this simple example, we studied multiple bases to sweeten a natural gas. We computed that temperature and pressure impacts H2S removal. The engineering piece would be layered on to this in order to design the most efficient scrubber.
If you wish to know more about the engineering chemistry of gas sweetening, feel free to contact us at [email protected] or visit our resource page www.olisystems.com/resources/

