One of OLI’s core sustainability missions is to provide solutions for carbon capture, transportation, utilization, and storage (CCTUS), with a focus on tackling corrosion issues in this domain. During our extensive collaboration with IFE, acid dropout within CO2 transportation assets has been identified as the potentially most severe threat to asset integrity. This is a complex phenomenon that can be understood using OLI’s solutions. With OLI’s expertise, we are committed to developing comprehensive solutions to address the formation of corrosive acidic phases, ensuring the long-term reliability of CCTUS infrastructure.
Figure 1 illustrates OLI’s comprehensive framework for modeling CCTUS asset integrity challenges. The foundation of OLI’s modeling framework is the accurate prediction for the thermodynamics of reactions between impurities and their impact on CO2 phase behavior. While the thermodynamic model identifies the worst-case scenario for acid formation and phase dropout, modeling of kinetically limited redox reactions need to be accounted for simultaneously. To complete the picture, an electrochemical corrosion kinetics model is essential to quantify the rate at which carbon steel deteriorates when exposed to such acidic phase.
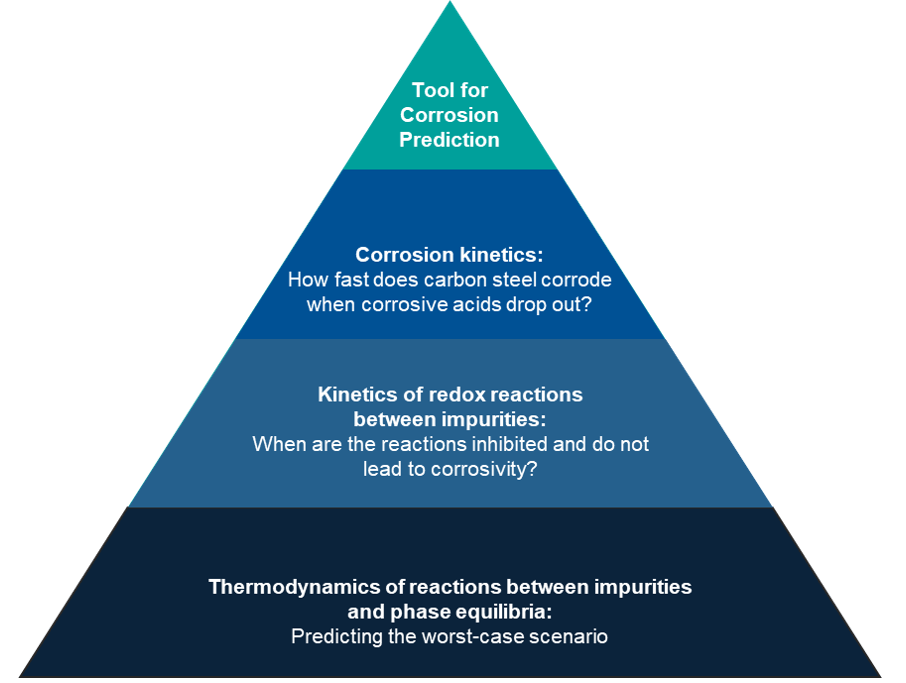
Figure 1. The OLI roadmap in developing a CO2 asset integrity tool to predict corrosion risks
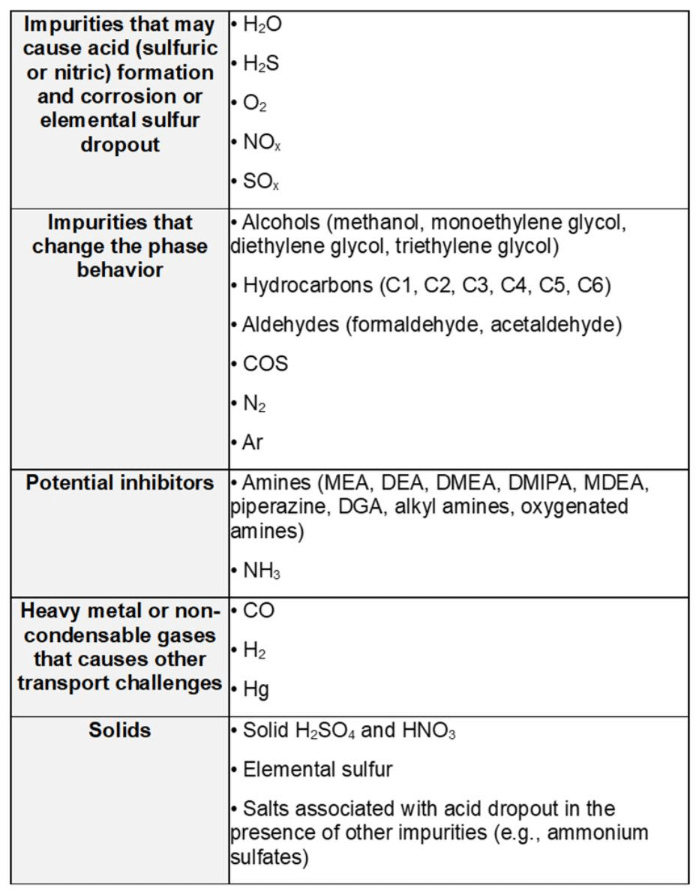
In this blog, we focus on the thermodynamic modeling of impurity reactions and phase equilibria, a critical aspect of understanding corrosion in CCTUS applications. The current version of OLI software (V12) supports a wide range of impurities that could pose operational challenges, particularly regarding corrosion within CCTUS applications. The MSE database incorporates various classes of impurities that can enter the CO2 stream across different sectors, including power plants, industrial facilities, and other sources that burn fossil fuels such as coal, natural gas, oil, or biomass. Impurities may also arise during pre- or post-combustion capture processes. These impurities include reactive and non-reactive gases, non-condensable gases, solids, alcohols, aldehydes, amines, and more. Certain components – such as solid acids, non-reactive gases (e.g., Ar, H₂, CO), and light hydrocarbon-acid interactions – are not yet included in V12 but are slated for incorporation in the upcoming release. Table 1 provides a summary of the CO2 impurities available in the MSE database.
Table 1. Status of CO2 impurities in MSE
Multiparameter equations of state such as GERG or EOS-CG can precisely predict the volumetric/thermal properties of multiphase CO2 systems, such as density, compressibility, heat capacity, PVT data, etc. Also, engineering equations of state such as . Acid condensation or precipitation, in the form of some dropouts in different sectors of CCTUS, poses a serious threat to asset integrity. These dropouts result from reactions between impurities, mainly SOx, NOx, H2O, O2, and H2S. Additionally, solidification of those acidic dropouts is expected to be feasible at low temperatures particularly under cryogenic transportation of chilled CO2 on ships. Such phenomena occur under difficult-to-predict conditions and the commonly used thermodynamic models cannot mimic the composition of those dropouts. This is where the MSE model demonstrates its strengths.
CO2 phase behavior shifted by impurities
CO2 captured from refineries and pre-combustion often contains non-condensable gas impurities, which are produced during processes such as oxy-fuel combustion, gasification of coal, amine scrubbing, flue gas desulfurization, etc. These light gases, with their very low critical points, can significantly change the phase transition boundaries from gas to supercritical liquid when they mixed with CO2 in substantial amounts. Their presence increases the vapor pressure and can complicate CO2 transportation. Figure 2 shows the MSE model projection and the impact of non-reactive gases on the phase behavior of CO2.
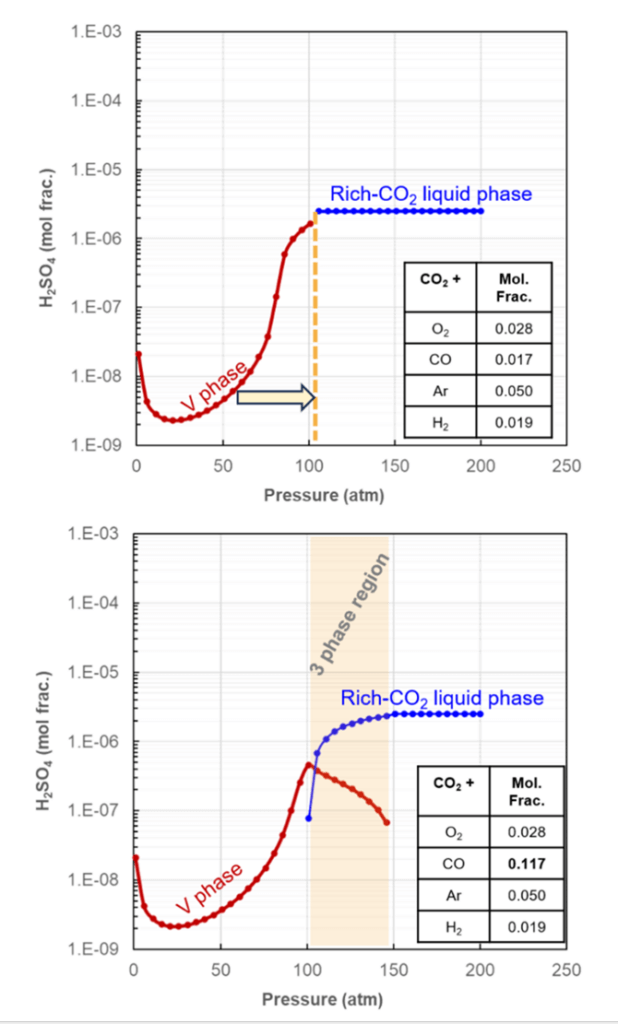
Figure 2: Examples of MSE model projection for sulfuric acid solubility in rich-CO2 phase with given impurity concentrations at 25°C
As shown in Figure 2a, the presence of impurities in CO2 might cause a pressure shift at which the acid solvation phase transitions from gas to CO2 -rich liquid. As predicted by the model, further contamination of the CO2 stream with CO (Figure 2b), can potentially create a three-phase stability region, where both gas and supercritical liquid CO2 coexist in equilibrium with an aqueous phase. This behavior can potentially complicate multiphase stability across various CCTUS sectors. Figure 3 presents another example of MSE model projections, showing the predicted occurrence of acid condensation or precipitation for a specific CO2 composition tested in Ref [1] under CO2 ship transportation.
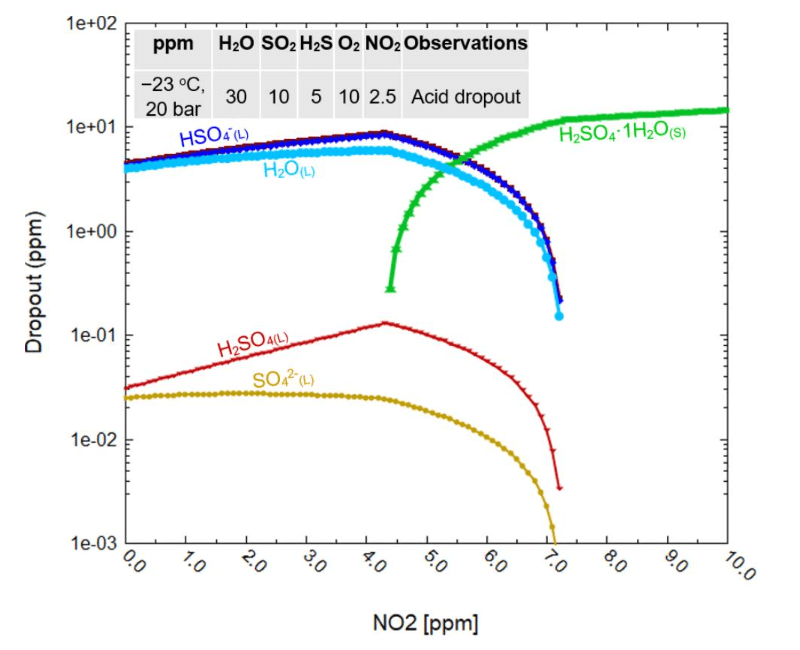
Figure 3: Examples of MSE model projection for phase composition of acid dropout during ship transport of CO2 (CO2 mixture composition/condition taken from Ref. 1)
As shown in Figure 3, MSE model predicts the liquid acid and water dropout at NO2 contents lower than about 7 ppm. Under these specific CO2 transportation conditions in ships, sulfate/bisulfate species are predicted to be the dominant acids formed in the liquid dropout, while nitric acid condensation is almost impossible. A significant water dropout in the liquid phase is also projected at NO2 levels lower than about 7 ppm. Water, even in very small amounts, can promote sweet corrosion, particularly localized attacks, under this particular transportation situation. Solid sulfuric acid monohydrate is also predicted to precipitate when NO2 contents are higher than about 4 ppm. This projection highlights conditions under which acidic species within a CO2-rich environment can condense or precipitate, forming liquid or solid dropouts, respectively, and potentially impacting operational stability and asset integrity by generating such corrosive phases. Even small quantities of NO2 impurities could jeopardize CO2 transport by facilitating the formation of an acidic phase that underscores the importance of establishing strict specifications for safe NO2 concentration limits in ship-based CO2 transportation.
Understanding these conditions is essential for preventing corrosion-related challenges due to the acid dropout and phase separation in CCTUS. For more information, or to consult and share ideas, feel free to contact us at https://www.olisystems.com/contact-us
References:
- Sonke, N.H. Morland, G. Moulie, M.S. Franke, Corrosion and chemical reactions in impure CO2, International Journal of Greenhouse Gas Control 133 (2024) 104075


