RESOURCES | post
Category: Water & Wastewater Treatment
What is arsenic and why is it important?
Being the 20th most abundant trace element in the Earth’s crust, arsenic (As) is widely distributed throughout the surface, ground waters as well as the biosphere and is involved in many geochemical processes and industrial activities, such as mineral dissolution, mineral processing, desorption in oxidizing environments and reductive desorption and dissolution. Arsenic is primarily used in various fields such as agriculture (pesticides), electronics (the second most commonly used semiconductor after doped silicon), wood preservation, metallurgy, and medicine [1]. These anthropogenic sources contribute to the release of arsenic to the environment in addition to its release from natural geological sources, for instance, by weathering of arsenic-containing rocks and volcanic activities. Arsenic release poses a threat to the environment and to human health.
Arsenic is known to be highly toxic in its inorganic form to all life forms [2]. It is one of World Health Organization’s (WHO) 10 chemicals of major public health concern and is a group 1 human carcinogenic substance [3]. Based on WHO guidelines, arsenic concentrations in drinking water should be strictly limited to 10 µg /L [4]. However, millions of people around the world are exposed to arsenic at concentrations much higher than the guideline value (100 µg /L or greater). In 2012, it was estimated that about 202 million people worldwide were exposed to arsenic concentrations in drinking water above 50 µg/L and this number substantially increased from 130 million in 2001. It is therefore a matter of urgency to prevent arsenic pollution. However, the strategies to avoid arsenic contamination and to develop environment-friendly processes of producing arsenic materials require novel techniques and innovative solutions.
Chemistry complexities and challenges
A knowledge of the speciation of As in water is important because the bioavailability and the physiological and toxicological effects of As depend on its chemical forms. Arsenic is a naturally occurring metalloid that is very mobile in the environment. Its mobility largely depends on the parent mineral form, oxidation state, and mobilization mechanisms, which result from many chemical and biological transformations in the aquatic environment. In terms of oxidation state, arsenic can exist in four forms, which are arsenite (As(III)), arsenate (As(V)), elemental arsenic (As(0)), and arsine (As(III)). Among these four arsenic species, the most prevalent forms, which are commonly found in water, are the inorganic arsenite and arsenate. Acids and metals, such as Ca, Fe, et al. play important roles in the removal of arsenic in different processes such as oxidation-reduction and precipitation-dissolution. Arsenic behavior in these processes is closely related to its chemical speciation and determines its removal efficiency. Understanding the geochemical processes which control the precipitation and dissolution of secondary As minerals in mine wastes is crucial for the formulation of models that predict the environmental impact of these sites.
OLI Systems simulation for arsenic removal
Quantitative geochemical calculations are not possible without accurate thermodynamic databases. “The creation of thermodynamic databases may be one of the greatest advances in the field of geochemistry of the last century [5].” OLI software V10 uses its proprietary Mixed Solvent Electrolyte (MSE) thermodynamic model, which includes the relevant acids, hydroxides, and various compounds of arsenic, including those with calcium, iron, carbonates and various complexing agents. The software enables scientific, data-driven, and reliable simulations that can improve the efficiency and environmental friendliness of arsenic removal processes.
Can we reliably predict the behavior of arsenic in aqueous systems?
In pyrometallurgical operations, arsenic becomes a problem in downstream treatment of the off-gases, in which it is present as arsenic trioxide (As2O3) vapor. However, the off-gases may also contain sulfur trioxide (SO3) which in the presence of water will form sulfuric acid (H2SO4). As the gas is cooled, acid will condense when the dew point of the gas is reached. Arsenic in acid at certain concentration forms crystals that can be separated from the acid. Therefore, accurate estimations of the thermodynamic properties of As2O3 and its solubility in H2SO4 solutions are essential for the optimal performance of the gas cleaning process.
The MSE thermodynamic framework in the OLI Software Platform V10 can accurately predict solubility of arsenic trioxide over wide ranges of temperature and concentration of H2SO4. The following figure shows the solubility of As2O3 in H2SO4 at different temperatures. The calculated solubilities are in good agreement with the experimental data.
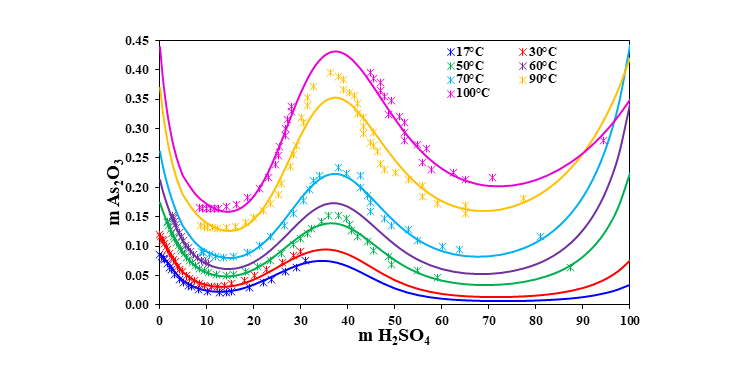
Solubility of As2O3 in H2SO4 at different temperatures
Lime (CaO) is widely used for treating industrial wastes. Reactions of calcium with arsenic oxyanions (AsO43-) form highly insoluble calcium arsenates to remove arsenic. The MSE model accurately predicts the formation of 4 precipitating solids, i.e., Ca3(AsO4)2·4.25H2O, Ca3(AsO4)2, Ca5(OH)(AsO4)3 and CaHAsO4·H2O. Not surprisingly, there is a good agreement between the predictions and experimental data.
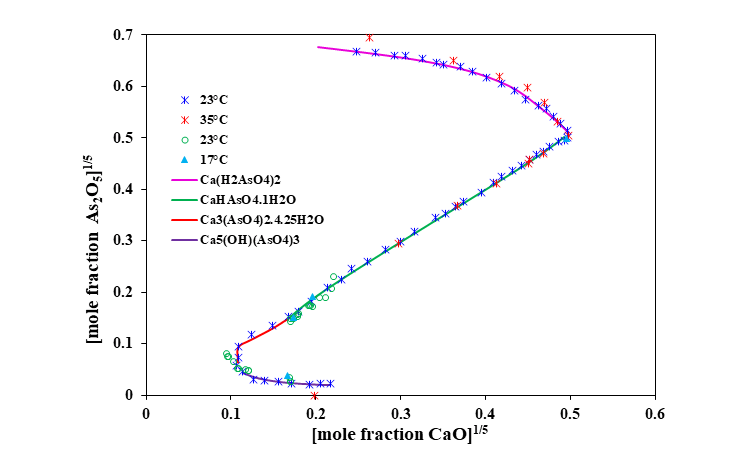
The ternary CaO-As2O5-H2O system
Iron arsenates, either well or poorly crystalline, are the usual phases of choice for arsenic immobilization in waste forms of variable origin. Iron is used for the immobilization of arsenic in industrial waste solutions for arsenic disposal. So, the compounds in the system Fe2O3-As2O5-H2O are common at hazardous waste sites such as mining and mineral processing facilities. Because poor crystallinity of Fe-As compounds, the chemical composition in terms of the Fe/As ratio and H2O content is highly variable. The MSE model shows that the As concentration can vary widely as a function of the nature, chemical composition, and crystallinity of these multicomponent oxides. In particular, the model can predict the aqueous As concentrations in systems in which scorodite (FeAsO4·2H2O) dissolves and ferric oxides precipitate.
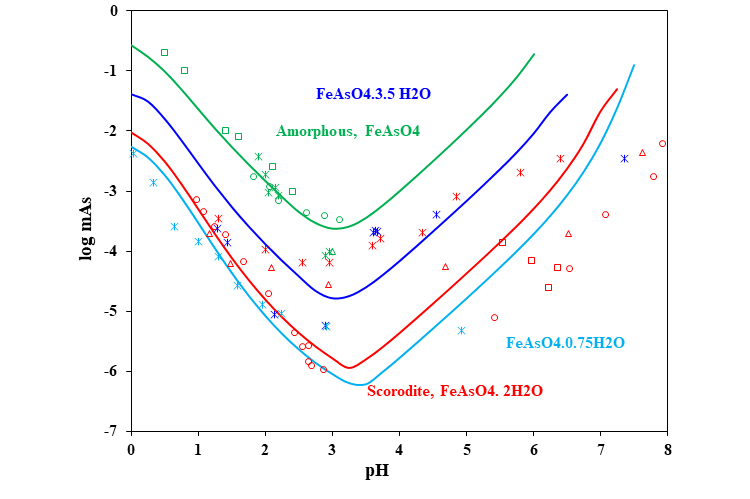
Solubility of scorodite in aqueous solution
Apart from the solubilities of arsenic-containing systems, the MSE model incorporated in the OLI Software Platform V10 can also calculate other thermodynamics properties such as vapor-liquid equilibria and caloric properties (heat capacities, heat of dilution) which are used to analyze and optimize streams in processes.
What tools are available for predicting arsenic removal?
The OLI System’s thermodynamic property package which is based on the MSE model, is available in OLI Studio V10 and OLI Flowsheet ESP V10.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- [1] Sharma, V.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Env. Int. 2009, 35, 743–759.
- [2] Singh, R.; Singh, S.; Parihar, P.; Singh, V.; Prasad, S. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270.
- [3] Van Halem, D.; Bakker, S.; Amy, G.; van Dijk, J. Arsenic in drinking water: A worldwide water quality concern for water supply companies. Drinking Water Eng. Sci. 2009, 2, 29–34.
- [4] Arsenic in Drinking Water; Organisation, W.H., Ed.; WHO: Geneva, Switzerland, 2011.
- [5] Oelkers EH, Benezeth P, Pokrovski GS (2009) Thermodynamic databases for w sulphuric acidater-rock interaction. In: Thermodynamics and Kinetics of Water-Rock Interaction. Oelkers EH, Schott J (eds) Rev Mineral Geochem 70:1-46
